The clinical value of patient-derived glioblastoma tumorspheres in predicting treatment response
- PMID: 28204560
- PMCID: PMC5737323
- DOI: 10.1093/neuonc/now304
The clinical value of patient-derived glioblastoma tumorspheres in predicting treatment response
Abstract
Background: Advances from glioma stemlike cell (GSC) research, though increasing our knowledge of glioblastoma (GBM) biology, do not influence clinical decisions yet. We explored the translational power of GSC-enriched cultures from patient-derived tumorspheres (TS) in predicting treatment response.
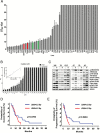
Methods: The relationship between TS growth and clinical outcome was investigated in 52 GBMs treated with surgical resection followed by radiotherapy and temozolomide (TMZ). The effect on TS of radiation (6 to 60 Gy) and of TMZ (3.9 μM to 1 mM) was related with patients' survival.
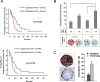
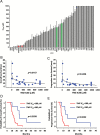
Results: Generation of TS was an independent factor for poor overall survival (OS) and poor progression-free survival (PFS) (P < .0001 and P = .0010, respectively). Growth rate and clonogenicity of TS predicted poor OS. In general, TS were highly resistant to both radiation and TMZ. Resistance to TMZ was stronger in TS with high clonogenicity and fast growth (P < .02). Shorter PFS was associated with radiation LD50 (lethal dose required to kill 50% of TS cells) >12 Gy of matched TS (P = .0484). A direct relationship was found between sensitivity of TS to TMZ and patients' survival (P = .0167 and P = .0436 for OS and PFS, respectively). Importantly, values for TMZ half-maximal inhibitory concentration <50 μM, which are in the range of plasma levels achieved in vivo, identified cases with longer OS and PFS (P = .0020 and P = .0016, respectively).
Conclusions: Analysis of TS holds translational relevance by predicting the response of parent tumors to radiation and, particularly, to TMZ. Dissecting the clonogenic population from proliferating progeny in TS can guide therapeutic strategies to a more effective drug selection and treatment duration.
Keywords: cancer stem cells; glioblastoma; radiotherapy; temozolomide; treatment outcome.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com
Figures


References
-
- Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. - PubMed
-
- Vermeulen L, de Sousa e Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncol. 2012;13(2):e83–e89. - PubMed
-
- Pallini R, Ricci-Vitiani L, Banna GL, et al. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14(24):8205–8212. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

