Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients
- PMID: 28205044
- PMCID: PMC5387027
- DOI: 10.1007/s10549-017-4155-2
Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients
Abstract
Background: Pathological complete response (pCR) is the ultimate response in breast cancer patients treated with neoadjuvant chemotherapy (NCT). It might be a surrogate outcome for disease-free survival (DFS) and overall survival (OS). We studied the effect of clinical tumor stage (cT-stage) on tumor pCR and the effect of pCR per cT-stage on 5-year OS and DFS.
Methods: Using the Netherlands Cancer Registry, all primary invasive breast cancer patients treated with NCT from 2005 until 2008 were identified. Univariable logistic regression analysis was performed to evaluate the effect of cT-stage on pCR, stepwise logistic regression analysis to correct for potential confounders and Kaplan-Meier survival analyses to calculate OS and DFS after five years.
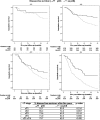
Results: In 2366 patients, overall pCR rate was 21%. For cT1, cT2, cT3, and cT4, pCR rates were 31, 22, 18, and 17%, respectively. Lower cT-stage (cT1-2 vs cT3-4) was a significant independent predictor of higher pCR rate (p < 0.001, OR 3.15). Furthermore, positive HER2 status (p < 0.001, OR 2.30), negative estrogen receptor status (p = 0.062, OR 1.69), and negative progesterone receptor status (p = 0.008, OR 2.27) were independent predictors of pCR. OS and DFS were up to 20% higher in patients with cT2-4 tumors with pCR versus patients without pCR. DFS was also higher for cT1 tumors with pCR.
Conclusions: The most important predictor of pCR in breast cancer patients is cT-stage: lower cT-stages have significantly higher pCR rates than higher cT-stages. Patients with cT2-4 tumors achieving pCR have higher OS and DFS compared to patients not achieving pCR.
Keywords: Breast cancer; Neoadjuvant chemotherapy; Pathological complete response; Survival; Tumor size.
Conflict of interest statement
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
We received all patient data in this study anonymously (without patient identifiers) from the Netherlands Cancer Registry (NCR). According to the Dutch law, all cancer patients are included in the NCR as maintained by the Netherlands Comprehensive Cancer Organisation (IKNL), unless the patient has objected to be registered. Therefore, informed consent was not applicable for this study.
Figures

References
-
- Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, Denkert C, Eiermann W, Gnant M, Harris JR, Karn T, Liedtke C, Mauri D, Rouzier R, Ruckhaeberle E, Semiglazov V, Symmans WF, Tutt A, Pusztai L. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19(5):1508–1516. doi: 10.1245/s10434-011-2108-2. - DOI - PubMed
-
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE, Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. - DOI - PubMed
-
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. - DOI - PubMed
-
- Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, Theoret H, Soran A, Wickerham DL, Wolmark N, National Surgical Adjuvant B, Bowel Project Protocol B (2003) The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 21(22):4165–4174. doi:10.1200/JCO.2003.12.005 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

