Systemically Infused Mesenchymal Stem Cells Show Different Homing Profiles in Healthy and Tumor Mouse Models
- PMID: 28205428
- PMCID: PMC5442841
- DOI: 10.1002/sctm.16-0204
Systemically Infused Mesenchymal Stem Cells Show Different Homing Profiles in Healthy and Tumor Mouse Models
Abstract
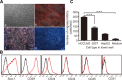
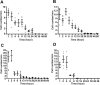
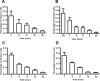
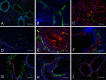
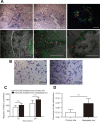
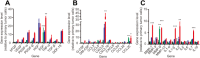
Bone marrow-derived mesenchymal stem cells (MSCs) can localize in injured, inflamed, and cancerous tissues after systemic infusion. However, the dynamic homing profile of MSCs in the peripheral blood is not well characterized. Here, using in vivo flow cytometry to noninvasively monitor the dynamics of fluorescence-labeled cells, we found different clearance kinetics of systemically infused MSCs between healthy and tumor mouse models. The circulation times of MSCs in healthy mice and mice with subcutaneous tumors, orthotopically transplanted liver tumors, or metastatic lung tumors were 30, 24, 18, and 12 hours, respectively, suggesting that MSCs actively home to tumor environments. MSCs infiltrated into hepatocellular carcinoma (HCC) sites and preferentially engrafted to micrometastatic regions both in vivo and in vitro. The expression of epidermal growth factor, CXCL9, CCL25, and matrix metalloproteinases-9 by HCC cells differed between primary tumor sites and metastatic regions. By characterizing the homing profiles of systemically perfused MSCs under physiological and cancerous conditions, these findings increase our understanding of the migration of MSCs from the circulation to tumor sites and constitute a basis for developing MSC-based anti-cancer therapeutic strategies. Stem Cells Translational Medicine 2017;6:1120-1131.
Keywords: Hepatocellular carcinoma; Homing; In vivo flow cytometry; Mesenchymal stem cells; Tumor xenograft model.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures
Similar articles
-
Characterization and Validation of Hepatocellular Carcinoma (HCC) Xenograft tumor as a Suitable Liver Cancer Model for Preclinical Mesenchymal Stem Cell Studies.Asian Pac J Cancer Prev. 2018 Jun 25;19(6):1627-1631. doi: 10.22034/APJCP.2018.19.6.1627. Asian Pac J Cancer Prev. 2018. PMID: 29936790 Free PMC article.
-
Combination therapy of sorafenib with mesenchymal stem cells as a novel cancer treatment regimen in xenograft models of hepatocellular carcinoma.J Cell Physiol. 2019 Jun;234(6):9495-9503. doi: 10.1002/jcp.27637. Epub 2018 Oct 26. J Cell Physiol. 2019. PMID: 30362607
-
Effect of bone-marrow-derived mesenchymal stem cells on high-potential hepatocellular carcinoma in mouse models: an intervention study.Eur J Med Res. 2013 Sep 30;18(1):34. doi: 10.1186/2047-783X-18-34. Eur J Med Res. 2013. PMID: 24079479 Free PMC article.
-
The therapeutic potential of bone marrow-derived mesenchymal stromal cells on hepatocellular carcinoma.Liver Int. 2014 Mar;34(3):330-42. doi: 10.1111/liv.12338. Epub 2013 Oct 21. Liver Int. 2014. PMID: 24112437 Review.
-
Mesenchymal stem cells as therapeutic tools and gene carriers in liver fibrosis and hepatocellular carcinoma.Gene Ther. 2010 Jun;17(6):692-708. doi: 10.1038/gt.2010.10. Epub 2010 Mar 11. Gene Ther. 2010. PMID: 20220785 Review.
Cited by
-
Mesenchymal Stem Cells in Gastric Cancer: Vicious but Hopeful.Front Oncol. 2021 May 11;11:617677. doi: 10.3389/fonc.2021.617677. eCollection 2021. Front Oncol. 2021. PMID: 34046337 Free PMC article. Review.
-
The role of mesenchymal stem cells in the occurrence, development, and therapy of hepatocellular carcinoma.Cancer Med. 2022 Feb;11(4):931-943. doi: 10.1002/cam4.4521. Epub 2022 Jan 3. Cancer Med. 2022. PMID: 34981659 Free PMC article. Review.
-
Stem cell therapy for hepatocellular carcinoma and end-stage liver disease.J Egypt Natl Canc Inst. 2023 Nov 6;35(1):35. doi: 10.1186/s43046-023-00194-z. J Egypt Natl Canc Inst. 2023. PMID: 37926787 Review.
-
Arming Mesenchymal Stromal/Stem Cells Against Cancer: Has the Time Come?Front Pharmacol. 2020 Sep 29;11:529921. doi: 10.3389/fphar.2020.529921. eCollection 2020. Front Pharmacol. 2020. PMID: 33117154 Free PMC article. Review.
-
Mesenchymal stem cell-delivered paclitaxel nanoparticles exhibit enhanced efficacy against a syngeneic orthotopic mouse model of pancreatic cancer.Int J Pharm. 2024 Dec 5;666:124753. doi: 10.1016/j.ijpharm.2024.124753. Epub 2024 Sep 24. Int J Pharm. 2024. PMID: 39321899
References
-
- Jemal A, Bray F, Center MM et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. - PubMed
-
- Schäfer M, Werner S. Cancer as an overhealing wound: An old hypothesis revisited. Nat Rev Mol Cell Biol 2008;9:628–638. - PubMed
-
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol Mech Dis 2006;1:119–150. - PubMed
-
- Erez N, Truitt M, Olson P et al. Cancer‐associated fibroblasts are activated in incipient neoplasia to orchestrate tumor‐promoting inflammation in an NF‐κB‐dependent manner. Cancer Cell 2010;17:135–147. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

