Proteins analysed as virtual knots
- PMID: 28205562
- PMCID: PMC5304221
- DOI: 10.1038/srep42300
Proteins analysed as virtual knots
Abstract
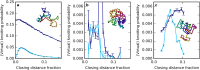
Long, flexible physical filaments are naturally tangled and knotted, from macroscopic string down to long-chain molecules. The existence of knotting in a filament naturally affects its configuration and properties, and may be very stable or disappear rapidly under manipulation and interaction. Knotting has been previously identified in protein backbone chains, for which these mechanical constraints are of fundamental importance to their molecular functionality, despite their being open curves in which the knots are not mathematically well defined; knotting can only be identified by closing the termini of the chain somehow. We introduce a new method for resolving knotting in open curves using virtual knots, which are a wider class of topological objects that do not require a classical closure and so naturally capture the topological ambiguity inherent in open curves. We describe the results of analysing proteins in the Protein Data Bank by this new scheme, recovering and extending previous knotting results, and identifying topological interest in some new cases. The statistics of virtual knots in protein chains are compared with those of open random walks and Hamiltonian subchains on cubic lattices, identifying a regime of open curves in which the virtual knotting description is likely to be important.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Knot localization in proteins.Biochem Soc Trans. 2013 Apr;41(2):538-41. doi: 10.1042/BST20120329. Biochem Soc Trans. 2013. PMID: 23514150 Review.
-
Subknots in ideal knots, random knots, and knotted proteins.Sci Rep. 2015 Mar 10;5:8928. doi: 10.1038/srep08928. Sci Rep. 2015. PMID: 25753957 Free PMC article.
-
Statistics of knots, geometry of conformations, and evolution of proteins.PLoS Comput Biol. 2006 May;2(5):e45. doi: 10.1371/journal.pcbi.0020045. Epub 2006 May 19. PLoS Comput Biol. 2006. PMID: 16710448 Free PMC article.
-
KnotProt: a database of proteins with knots and slipknots.Nucleic Acids Res. 2015 Jan;43(Database issue):D306-14. doi: 10.1093/nar/gku1059. Epub 2014 Oct 31. Nucleic Acids Res. 2015. PMID: 25361973 Free PMC article.
-
Protein knots and fold complexity: some new twists.Comput Biol Chem. 2007 Jun;31(3):151-62. doi: 10.1016/j.compbiolchem.2007.03.002. Epub 2007 Mar 24. Comput Biol Chem. 2007. PMID: 17500039 Review.
Cited by
-
Topological Models for Open-Knotted Protein Chains Using the Concepts of Knotoids and Bonded Knotoids.Polymers (Basel). 2017 Sep 13;9(9):444. doi: 10.3390/polym9090444. Polymers (Basel). 2017. PMID: 30965745 Free PMC article.
-
Is There a Functional Role for the Knotted Topology in Protein UCH-L1?J Chem Inf Model. 2024 Sep 9;64(17):6827-6837. doi: 10.1021/acs.jcim.4c00880. Epub 2024 Jul 24. J Chem Inf Model. 2024. PMID: 39045738 Free PMC article.
-
Studies of global and local entanglements of individual protein chains using the concept of knotoids.Sci Rep. 2017 Jul 24;7(1):6309. doi: 10.1038/s41598-017-06649-3. Sci Rep. 2017. PMID: 28740166 Free PMC article.
-
To Tie or Not to Tie? That Is the Question.Polymers (Basel). 2017 Sep 16;9(9):454. doi: 10.3390/polym9090454. Polymers (Basel). 2017. PMID: 30965758 Free PMC article. Review.
-
Topological descriptions of protein folding.Proc Natl Acad Sci U S A. 2019 May 7;116(19):9360-9369. doi: 10.1073/pnas.1808312116. Epub 2019 Apr 18. Proc Natl Acad Sci U S A. 2019. PMID: 31000594 Free PMC article.
References
-
- Branden C. I. & Tooze J. Introduction to Protein Structure. chap. 1 (Garland Science, 1998).
-
- Taylor W. R. A deeply knotted protein structure and how it might fold. Nature 406, 916–9 (2000). - PubMed
-
- Millett K. C., Rawdon E. J., Stasiak A. & Sulkowska J. L. Identifying knots in proteins. Biochemical Society Transactions 41, 533–7 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

