CuII(atsm) improves the neurological phenotype and survival of SOD1G93A mice and selectively increases enzymatically active SOD1 in the spinal cord
- PMID: 28205575
- PMCID: PMC5304223
- DOI: 10.1038/srep42292
CuII(atsm) improves the neurological phenotype and survival of SOD1G93A mice and selectively increases enzymatically active SOD1 in the spinal cord
Abstract
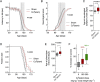
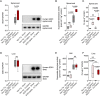
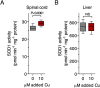
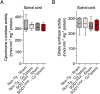
Ubiquitous expression of mutant Cu/Zn-superoxide dismutase (SOD1) selectively affects motor neurons in the central nervous system (CNS), causing the adult-onset degenerative disease amyotrophic lateral sclerosis (ALS). The CNS-specific impact of ubiquitous mutant SOD1 expression is recapitulated in transgenic mouse models of the disease. Here we present outcomes for the metallo-complex CuII(atsm) tested for therapeutic efficacy in mice expressing SOD1G93A on a mixed genetic background. Oral administration of CuII(atsm) delayed the onset of neurological symptoms, improved locomotive capacity and extended overall survival. Although the ALS-like phenotype of SOD1G93A mice is instigated by expression of the mutant SOD1, we show the improved phenotype of the CuII(atsm)-treated animals involves an increase in mature mutant SOD1 protein in the disease-affected spinal cord, where concomitant increases in copper and SOD1 activity are also evident. In contrast to these effects in the spinal cord, treating with CuII(atsm) had no effect in liver on either mutant SOD1 protein levels or its activity, indicating a CNS-selective SOD1 response to the drug. These data provide support for CuII(atsm) as a treatment option for ALS as well as insight to the CNS-selective effects of mutant SOD1.
Conflict of interest statement
Collaborative Medicinal Development has licensed IP pertaining to CuII(atsm) from the University of Melbourne where the inventors include ARW and PSD.
Figures
References
-
- Rosen D. R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993). - PubMed
-
- Gurney M. E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994). - PubMed
-
- Wong P. C. et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14, 1105–1116 (1995). - PubMed
-
- Gingras B. A., Suprunchuk T. & Bayley C. H. The preparation of some thiosemicarbazones and their copper complexes: Part III. Can. J. Chem. 40, 1053–1059 (1962).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

