Comparative studies of oxaliplatin-based platinum(iv) complexes in different in vitro and in vivo tumor models
- PMID: 28205649
- PMCID: PMC6130776
- DOI: 10.1039/c6mt00226a
Comparative studies of oxaliplatin-based platinum(iv) complexes in different in vitro and in vivo tumor models
Abstract
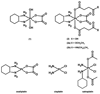
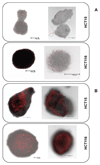
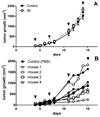
Using platinum(iv) prodrugs of clinically established platinum(ii) compounds is a strategy to overcome side effects and acquired resistances. We studied four oxaliplatin-derived platinum(iv) complexes with varying axial ligands in various in vitro and in vivo settings. The ability to interfere with DNA (pUC19) in the presence and absence of a reducing agent (ascorbic acid) was investigated in cell-free experiments. Cytotoxicity was compared under normoxic and hypoxic conditions in monolayer cultures and multicellular spheroids of colon carcinoma cell lines. Effects on the cell cycle were investigated by flow cytometry, and the capacity of inducing apoptosis was confirmed by flow cytometry and Western blotting. The anti-cancer activity of one complex was studied in vivo in immunodeficient and immunocompetent mice, and the platinum levels in various organs and the tumor after treatment were quantified. The results demonstrate that modification of the axial ligands can improve the cytotoxic potency. The complexes are able to interfere with plasmid DNA, which is enhanced by co-incubation with a reducing agent, and cause cell cycle perturbations. At higher concentrations, they induce apoptosis, but generate only low levels of reactive oxygen species. Two of the complexes increase the life span of leukemia (L1210) bearing mice, and one showed effects similar to oxaliplatin in a CT26 solid tumor model, despite the low platinum levels in the tumor. As in the case of oxaliplatin, activity in the latter model depends on an intact immune system. These findings show new perspectives for the development of platinum(iv) prodrugs of the anticancer agent oxaliplatin, combining bioreductive properties and immunogenic aspects.
Conflict of interest statement
The authors declare no conflict of interests.
Figures




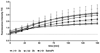


References
-
- Reedijk J. Increased understanding of platinum anticancer chemistry. Pure Appl Chem. 2011;83:1709–1719.
-
- Wheate NJ, Walker S, Craig GE, Oun R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010;39:8113–8127. - PubMed
-
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. - PubMed
-
- Jung Y, Lippard SJ. Direct cellular responses to platinum-induced DNA damage. Chem Rev. 2007;107:1387–1407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

