Aspartate Glycosylation Triggers Isomerization to Isoaspartate
- PMID: 28207246
- PMCID: PMC5431074
- DOI: 10.1021/jacs.6b12866
Aspartate Glycosylation Triggers Isomerization to Isoaspartate
Abstract
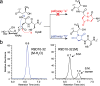
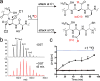
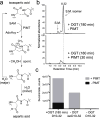
O-Linked β-N-acetylglucosamine transferase (OGT) is an essential human enzyme that glycosylates numerous nuclear and cytoplasmic proteins on serine and threonine. It also cleaves Host cell factor 1 (HCF-1) by a mechanism in which the first step involves glycosylation on glutamate. Replacing glutamate with aspartate in an HCF-1 proteolytic repeat was shown to prevent peptide backbone cleavage, but whether aspartate glycosylation occurred was not examined. We report here that OGT glycosylates aspartate much faster than it glycosylates glutamate in an otherwise identical model peptide substrate; moreover, once formed, the glycosyl aspartate reacts further to form a succinimide intermediate that hydrolyzes to produce the corresponding isoaspartyl peptide. Aspartate-to-isoaspartate isomerization in proteins occurs in cells but was previously thought to be exclusively non-enzymatic. Our findings suggest it may also be enzyme-catalyzed. In addition to OGT, enzymes that may catalyze aspartate to isoaspartate isomerization include PARPs, enzymes known to ribosylate aspartate residues in the process of poly(ADP-ribosyl)ation.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Capotosti F, Guernier S, Lammers F, Waridel P, Cai Y, Jin J, Conaway JW, Conaway RC, Herr W. Cell. 2011;144:376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

