Incidence and risk factors of acute kidney injury associated with continuous intravenous high-dose vancomycin in critically ill patients: A retrospective cohort study
- PMID: 28207512
- PMCID: PMC5319501
- DOI: 10.1097/MD.0000000000006023
Incidence and risk factors of acute kidney injury associated with continuous intravenous high-dose vancomycin in critically ill patients: A retrospective cohort study
Abstract
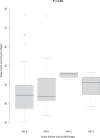
For vancomycin therapy of severe infections, the Infectious Diseases Society of America recommends high vancomycin trough levels, whose potential for inducing nephrotoxicity is controversial. We evaluated the incidence and risk factors of acute kidney injury (AKI) in critically ill patients given continuous intravenous vancomycin with target serum vancomycin levels of 20 to 30 mg/L.We retrospectively studied 107 continuous intravenous vancomycin treatments of ≥48 hours' duration with at least 2 serum vancomycin levels ≥20 mg/L in critically ill patients. Nephrotoxicity was defined according to the Kidney Disease Improving Global Outcomes Clinical Practice Guideline for AKI (ie, serum creatinine elevation by ≥26.5 μmoL/L or to ≥1.5 times baseline). Risk factors for AKI were identified by univariate and multivariate analyses.AKI developed in 31 (29%) courses. Higher serum vancomycin levels were associated with AKI (P < 0.01). Factors independently associated with AKI were highest serum vancomycin ≥40 mg/L (odds ratio [OR], 3.75; 95% confidence interval [CI], 1.40-10.37; P < 0.01), higher cumulative number of organ failures (OR, 2.63 95%CI, 1.42-5.31; P < 0.01), and cirrhosis of the liver (OR, 5.58; 95%CI, 1.08-31.59; P = 0.04).In this study, 29% of critically ill patients had AKI develop during continuous intravenous vancomycin therapy targeting serum levels of 20 to 30 mg/L. Serum vancomycin level ≥40 mg/L was independently associated with AKI.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- Levine DP. Vancomycin: a history. Clin Infect Dis Off Publ Infect Dis Soc Am 2006;42suppl 1:S5–12. - PubMed
-
- Rybak MJ, Lomaestro BM, Rotschafer JC. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis Off Publ Infect Dis Soc Am 2009;49:325–7. - PubMed
-
- Kullar R, Davis SL, Levine DP, et al. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis 2011;52:975–81. - PubMed
-
- Liu C, Bayer A, Cosgrove SE. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011;52:e18–55. - PubMed
-
- Bingen E, Mariani-Kurkdjian P, Nebbad B. Optimal vancomycin serum level in Staphylococcus aureus infections? Médecine Mal Infect 2006;36:439–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

