Earlier Endpoints are Required for Hemorrhagic Shock Trials Among Severely Injured Patients
- PMID: 28207628
- PMCID: PMC5392160
- DOI: 10.1097/SHK.0000000000000788
Earlier Endpoints are Required for Hemorrhagic Shock Trials Among Severely Injured Patients
Abstract
Background: Choosing the appropriate endpoint for a trauma hemorrhage control trial can determine the likelihood of its success. Recent Phase 3 trials and observational studies have used 24-h and/or 30-day all-cause mortality as the primary endpoint and some have not used exception from informed consent (EFIC), resulting in multiple failed trials. Five recent high-quality prospective studies among 4,064 hemorrhaging trauma patients provide new evidence to support earlier primary endpoints.
Methods: The goal of this project was to determine the optimal endpoint for hemorrhage control trials using existing literature and new analyses of previously published data.
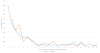
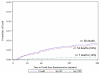
Results: Recent studies among bleeding trauma patients show that hemorrhagic deaths occur rapidly, at a high rate, and in a consistent pattern. Early preventable deaths among trauma patients are largely due to hemorrhage and the median time to hemorrhagic death from admission is 2.0 to 2.6 h. Approximately 85% of hemorrhagic deaths occur within 6 h. The hourly mortality rate due to traumatic injury decreases rapidly after enrollment from 4.6% per hour at 1 hour postenrollment to 1% per hour at 6 h to <0.1% per hour by 9 h and thereafter. Early primary endpoints (within 6 h) have critically important benefits for hemorrhage control trials, including being congruent with the median time to hemorrhagic death, biologic plausibility, and enabling the use of all-cause mortality, which is definitive and objective.
Conclusions: Primary endpoints should be congruent with the timing of the disease process. Therefore, if a resuscitation/hemorrhage control intervention is under study, a primary endpoint of all-cause mortality evaluated within the first 6 h is appropriate. Before choosing the timing of the primary endpoint for a large multicenter trial, we recommend performing a Phase 2 trial under EFIC to better understand the effects of the hemorrhage control intervention and distribution of time to death. When early primary endpoints are used, patients should be monitored for multiple subsequent secondary safety endpoints, including 24 h and 30-day all-cause mortality as well as the customary safety endpoints.
Figures



References
-
- National Academies of Sciences, Engineering, and Medicine . A national trauma care system: Integrating military and civilian trauma systems to achieve zero preventable deaths after injury. The National Academies Press; Washington, DC: 2016. - PubMed
-
- Norton R, Kobusingye O. Injuries. N Engl J Med. 2013;368(18):1723–1730. - PubMed
-
- Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13–21. - PubMed
-
- Kwon AM, Garbett NC, Kloecker GH. Pooled preventable death rates in trauma patients : Meta analysis and systematic review since 1990. Eur J Trauma Emerg Surg. 2014;40(3):279–285. - PubMed
-
- Holcomb JB, Weiskopf R, Champion H, Gould SA, Sauer RM, Brasel K, Bochicchio G, Bulger E, Cotton BA, Davis D, et al. Challenges to effective research in acute trauma resuscitation: consent and endpoints. Shock. 2011;35(2):107–113. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

