Update: Influenza Activity - United States, October 2, 2016-February 4, 2017
- PMID: 28207684
- PMCID: PMC5657859
- DOI: 10.15585/mmwr.mm6606a2
Update: Influenza Activity - United States, October 2, 2016-February 4, 2017
Abstract
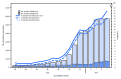
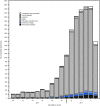
This report summarizes U.S. influenza activity* during October 2, 2016-February 4, 2017,† and updates the previous summary (1). Influenza activity in the United States began to increase in mid-December, remained elevated through February 4, 2017, and is expected to continue for several more weeks. To date, influenza A (H3N2) viruses have predominated overall, but influenza A (H1N1)pdm09 and influenza B viruses have also been identified.
Figures

References
-
- Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011;60(No. RR-1). - PubMed
-
- Food and Drug Administration. The FDA approves first generic version of widely used influenza drug, Tamiflu. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2016. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPa...
-
- New York City Department of Health and Mental Hygiene. Health department investigation on H7N2 influenza in shelter cats confirms risk to humans is low. New York, NY: New York City Department of Health and Mental Hygiene; 2016. http://www1.nyc.gov/site/doh/about/press/pr2016/pr107-16.page
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

