Analyzing pepsin degradation assay conditions used for allergenicity assessments to ensure that pepsin susceptible and pepsin resistant dietary proteins are distinguishable
- PMID: 28207780
- PMCID: PMC5312868
- DOI: 10.1371/journal.pone.0171926
Analyzing pepsin degradation assay conditions used for allergenicity assessments to ensure that pepsin susceptible and pepsin resistant dietary proteins are distinguishable
Abstract
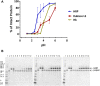
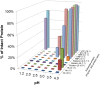
The susceptibility of a dietary protein to proteolytic degradation by digestive enzymes, such as gastric pepsin, provides information on the likelihood of systemic exposure to a structurally intact and biologically active macromolecule, thus informing on the safety of proteins for human and animal consumption. Therefore, the purpose of standardized in vitro degradation studies that are performed during protein safety assessments is to distinguish whether proteins of interest are susceptible or resistant to pepsin degradation via a study design that enables study-to-study comparison. Attempting to assess pepsin degradation under a wide-range of possible physiological conditions poses a problem because of the lack of robust and consistent data collected under a large-range of sub-optimal conditions, which undermines the needs to harmonize in vitro degradation conditions. This report systematically compares the effects of pH, incubation time, and pepsin-to-substrate protein ratio on the relative degradation of five dietary proteins: three pepsin susceptible proteins [ribulose 1,5-bisphosphate carboxylase-oxygenase (Rubisco), horseradish peroxidase (HRP), hemoglobin (Hb)], and two pepsin resistant proteins [lipid transfer protein (LTP) and soybean trypsin inhibitor (STI)]. The results indicate that proteins susceptible to pepsin degradation are readily distinguishable from pepsin-resistant proteins when the reaction conditions are within the well-characterized optima for pepsin. The current standardized in vitro pepsin resistant assay with low pH and high pepsin-to-substrate ratio fits this purpose. Using non-optimal pH and/or pepsin-to-substrate protein ratios resulted in susceptible proteins no longer being reliably degraded by this stomach enzyme, which compromises the ability of this in vitro assay to distinguish between resistant and susceptible proteins and, therefore, no longer providing useful data to an overall weight-of-evidence approach to assessing safety of proteins.
Conflict of interest statement
Figures



Similar articles
-
A multi-laboratory evaluation of a common in vitro pepsin digestion assay protocol used in assessing the safety of novel proteins.Regul Toxicol Pharmacol. 2004 Apr;39(2):87-98. doi: 10.1016/j.yrtph.2003.11.003. Regul Toxicol Pharmacol. 2004. PMID: 15041142
-
Relationship between protein digestibility and allergenicity: comparisons of pepsin and cathepsin.Toxicology. 2013 Jul 5;309:30-8. doi: 10.1016/j.tox.2013.04.011. Epub 2013 Apr 23. Toxicology. 2013. PMID: 23624183
-
Effect of initial protein concentration and pH on in vitro gastric digestion of heated whey proteins.Food Chem. 2014 Feb 15;145:473-80. doi: 10.1016/j.foodchem.2013.08.076. Epub 2013 Aug 29. Food Chem. 2014. PMID: 24128503
-
Food Allergens: Is There a Correlation between Stability to Digestion and Allergenicity?Crit Rev Food Sci Nutr. 2016 Jul 3;56(9):1545-67. doi: 10.1080/10408398.2013.779569. Crit Rev Food Sci Nutr. 2016. PMID: 25607526 Review.
-
Impact of gastric pH profiles on the proteolytic digestion of mixed βlg-Xanthan biopolymer gels.Food Funct. 2016 Jan;7(1):58-68. doi: 10.1039/c5fo01085c. Food Funct. 2016. PMID: 26599197 Review.
Cited by
-
Statement on in vitro protein digestibility tests in allergenicity and protein safety assessment of genetically modified plants.EFSA J. 2021 Jan 12;19(1):e06350. doi: 10.2903/j.efsa.2021.6350. eCollection 2021 Jan. EFSA J. 2021. PMID: 33473251 Free PMC article.
-
Comparative study of the binding between chlorogenic acid and four proteins by isothermal titration calorimetry, spectroscopy and docking methods.Pharmacol Rep. 2022 Jun;74(3):523-538. doi: 10.1007/s43440-022-00369-w. Epub 2022 May 11. Pharmacol Rep. 2022. PMID: 35545727
-
Non-natural amino acids into LfcinB-derived peptides: effect in their (i) proteolytic degradation and (ii) cytotoxic activity against cancer cells.R Soc Open Sci. 2023 Jun 14;10(6):221493. doi: 10.1098/rsos.221493. eCollection 2023 Jun. R Soc Open Sci. 2023. PMID: 37325596 Free PMC article.
-
Presence of small resistant peptides from new in vitro digestion assays detected by liquid chromatography tandem mass spectrometry: An implication of allergenicity prediction of novel proteins?PLoS One. 2020 Jun 15;15(6):e0233745. doi: 10.1371/journal.pone.0233745. eCollection 2020. PLoS One. 2020. PMID: 32542029 Free PMC article.
-
Scientific Opinion on development needs for the allergenicity and protein safety assessment of food and feed products derived from biotechnology.EFSA J. 2022 Jan 25;20(1):e07044. doi: 10.2903/j.efsa.2022.7044. eCollection 2022 Jan. EFSA J. 2022. PMID: 35106091 Free PMC article.
References
-
- Caspary WF. Physiology and pathophysiology of intestinal absorption. The Am J Nutr 1992; 55(1 Suppl):299s–308s. - PubMed
-
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 2008.
-
- Antonov VK, Rumsh LD, Tikhodeeva AG. Kinetics of pepsin-catalysed transpeptidation: Evidence for the ‘amino-enzyme’ intermediate. FEBS Lett 1974;46(1): 29–33. - PubMed
-
- Gardner ML. Gastrointestinal absorption of intact proteins. AnnuRev Nutr 1988; 8:329–50. - PubMed
-
- CODEX ALIMENTARIUS. Foods derived from modern biotechnology 2009. ftp://ftp.fao.org/codex/Publications/Booklets/Biotech/Biotech_2009e.pdf
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

