Differential effects of silencing crustacean hyperglycemic hormone gene expression on the metabolic profiles of the muscle and hepatopancreas in the crayfish Procambarus clarkii
- PMID: 28207859
- PMCID: PMC5313166
- DOI: 10.1371/journal.pone.0172557
Differential effects of silencing crustacean hyperglycemic hormone gene expression on the metabolic profiles of the muscle and hepatopancreas in the crayfish Procambarus clarkii
Abstract
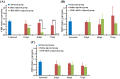
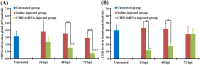
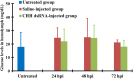
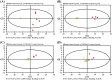
In order to functionally characterize the metabolic roles of crustacean hyperglycemic hormone (CHH), gene expression of CHH in the crayfish (Procambarus clarkii) was knocked down by in vivo injection of CHH double-stranded RNA (dsRNA), followed by metabolomic analysis of 2 CHH target tissues (the muscle and hepatopancreas) using nuclear magnetic resonance spectroscopy. Compared to the levels in untreated and saline-injected (SAI) animals, levels of CHH transcript, but not those of molt-inhibiting hormone (a CHH-family peptide), in the eyestalk ganglia of CHH dsRNA-injected (DSI) animals were significantly decreased at 24, 48, and 72 hour post injection (hpi), with concomitant changes in levels of CHH peptide in the sinus gland (a neurohemal organ) and hemolymph. Green fluorescence protein (GFP) dsRNA failed to affect levels of CHH transcript in the eyestalk ganglia of GFP DSI animals. Number of metabolites whose levels were significantly changed by CHH dsRNA was 149 and 181 in the muscle and 24 and 12 in the hepatopancreas, at 24 and 48 hpi, respectively. Principal component analysis of these metabolites show that metabolic effects of silencing CHH gene expression were more pronounced in the muscle (with the cluster of CHH DSI group clearly being separated from that of SAI group at 24 hpi) than in the hepatopancreas. Moreover, pathway analysis of the metabolites closely related to carbohydrate and energy metabolism indicate that, for CHH DSI animals at 24 hpi, metabolic profile of the muscle was characterized by reduced synthesis of NAD+ and adenine ribonucleotides, diminished levels of ATP, lower rate of utilization of carbohydrates through glycolysis, and a partially rescued TCA cycle, whereas that of the hepatopancreas by unaffected levels of ATP, lower rate of utilization of carbohydrates, and increased levels of ketone bodies. The combined results of metabolic changes in response to silenced CHH gene expression reveal that metabolic functions of CHH on the muscle and hepatopancreas are more diverse than previously thought and are differential between the two tissues.
Conflict of interest statement
Figures
Similar articles
-
Regulation of amino acid and nucleotide metabolism by crustacean hyperglycemic hormone in the muscle and hepatopancreas of the crayfish Procambarus clarkia.PLoS One. 2019 Dec 26;14(12):e0221745. doi: 10.1371/journal.pone.0221745. eCollection 2019. PLoS One. 2019. PMID: 31877133 Free PMC article.
-
The Crustacean Hyperglycemic Hormone Superfamily: Progress Made in the Past Decade.Front Endocrinol (Lausanne). 2020 Oct 1;11:578958. doi: 10.3389/fendo.2020.578958. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33117290 Free PMC article. Review.
-
Neuroendocrine responses of a crustacean host to viral infection: effects of infection of white spot syndrome virus on the expression and release of crustacean hyperglycemic hormone in the crayfish Procambarus clarkii.Comp Biochem Physiol A Mol Integr Physiol. 2013 Feb;164(2):327-32. doi: 10.1016/j.cbpa.2012.11.009. Epub 2012 Nov 19. Comp Biochem Physiol A Mol Integr Physiol. 2013. PMID: 23174320
-
Crustacean hyperglycemic hormone is synthesized in the eyestalk and brain of the crayfish Procambarus clarkii.PLoS One. 2017 Apr 3;12(4):e0175046. doi: 10.1371/journal.pone.0175046. eCollection 2017. PLoS One. 2017. PMID: 28369112 Free PMC article.
-
Crustacean hyperglycemic hormone (CHH) neuropeptidesfamily: Functions, titer, and binding to target tissues.Gen Comp Endocrinol. 2010 May 1;166(3):447-54. doi: 10.1016/j.ygcen.2009.12.011. Epub 2009 Dec 22. Gen Comp Endocrinol. 2010. PMID: 20026335 Review.
Cited by
-
Silencing of crustacean hyperglycemic hormone gene expression reveals the characteristic energy and metabolic changes in the gills and epidermis of crayfish Procambarus clarkii.Front Physiol. 2024 Jan 10;14:1349106. doi: 10.3389/fphys.2023.1349106. eCollection 2023. Front Physiol. 2024. PMID: 38269063 Free PMC article.
-
Regulation of amino acid and nucleotide metabolism by crustacean hyperglycemic hormone in the muscle and hepatopancreas of the crayfish Procambarus clarkia.PLoS One. 2019 Dec 26;14(12):e0221745. doi: 10.1371/journal.pone.0221745. eCollection 2019. PLoS One. 2019. PMID: 31877133 Free PMC article.
-
Structure-Based Functional Analysis of a Hormone Belonging to an Ecdysozoan Peptide Superfamily: Revelation of a Common Molecular Architecture and Residues Possibly for Receptor Interaction.Int J Mol Sci. 2021 Oct 15;22(20):11142. doi: 10.3390/ijms222011142. Int J Mol Sci. 2021. PMID: 34681803 Free PMC article.
-
The Crustacean Hyperglycemic Hormone Superfamily: Progress Made in the Past Decade.Front Endocrinol (Lausanne). 2020 Oct 1;11:578958. doi: 10.3389/fendo.2020.578958. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33117290 Free PMC article. Review.
References
-
- Cooke IM, Sullivan RE. Hormones and neurosecretion In: Atwood HL, Sandeman DC, editors. New York: Academic Press; The Biology of Crustacea. 1982;3:206–290.
-
- Keller R. Crustacean neuropeptides: structures, functions, and comparative aspects. Experientia. 1992;48:439–448. - PubMed
-
- Soyez D. Occurrence and diversity of neuropeptides from the crustacean hyperglycemic hormone family in arthropods. Ann N Y Acad Sci. 1997;814:319–323. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

