Skeletal muscle metabolic adaptations to endurance exercise training are attainable in mice with simvastatin treatment
- PMID: 28207880
- PMCID: PMC5313210
- DOI: 10.1371/journal.pone.0172551
Skeletal muscle metabolic adaptations to endurance exercise training are attainable in mice with simvastatin treatment
Abstract
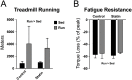
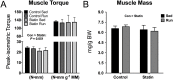
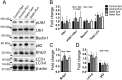
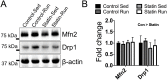
We tested the hypothesis that a 6-week regimen of simvastatin would attenuate skeletal muscle adaptation to low-intensity exercise. Male C57BL/6J wildtype mice were subjected to 6-weeks of voluntary wheel running or normal cage activities with or without simvastatin treatment (20 mg/kg/d, n = 7-8 per group). Adaptations in in vivo fatigue resistance were determined by a treadmill running test, and by ankle plantarflexor contractile assessment. The tibialis anterior, gastrocnemius, and plantaris muscles were evaluated for exercised-induced mitochondrial adaptations (i.e., biogenesis, function, autophagy). There was no difference in weekly wheel running distance between control and simvastatin-treated mice (P = 0.51). Trained mice had greater treadmill running distance (296%, P<0.001), and ankle plantarflexor contractile fatigue resistance (9%, P<0.05) compared to sedentary mice, independent of simvastatin treatment. At the cellular level, trained mice had greater mitochondrial biogenesis (e.g., ~2-fold greater PGC1α expression, P<0.05) and mitochondrial content (e.g., 25% greater citrate synthase activity, P<0.05), independent of simvastatin treatment. Mitochondrial autophagy-related protein contents were greater in trained mice (e.g., 40% greater Bnip3, P<0.05), independent of simvastatin treatment. However, Drp1, a marker of mitochondrial fission, was less in simvastatin treated mice, independent of exercise training, and there was a significant interaction between training and statin treatment (P<0.022) for LC3-II protein content, a marker of autophagy flux. These data indicate that whole body and skeletal muscle adaptations to endurance exercise training are attainable with simvastatin treatment, but simvastatin may have side effects on muscle mitochondrial maintenance via autophagy, which could have long-term implications on muscle health.
Conflict of interest statement
Figures


Similar articles
-
Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance.FASEB J. 2013 Oct;27(10):4184-93. doi: 10.1096/fj.13-228486. Epub 2013 Jun 27. FASEB J. 2013. PMID: 23825228 Free PMC article.
-
Exercise training improves plantar flexor muscle function in mdx mice.Med Sci Sports Exerc. 2012 Sep;44(9):1671-9. doi: 10.1249/MSS.0b013e31825703f0. Med Sci Sports Exerc. 2012. PMID: 22460476 Free PMC article.
-
Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition.J Physiol Sci. 2016 Sep;66(5):417-30. doi: 10.1007/s12576-016-0440-9. Epub 2016 Mar 4. J Physiol Sci. 2016. PMID: 26943341 Free PMC article.
-
Adaptations of skeletal muscle to prolonged, intense endurance training.Clin Exp Pharmacol Physiol. 2002 Mar;29(3):218-22. doi: 10.1046/j.1440-1681.2002.03623.x. Clin Exp Pharmacol Physiol. 2002. PMID: 11906487 Review.
-
Transient changes to metabolic homeostasis initiate mitochondrial adaptation to endurance exercise.Semin Cell Dev Biol. 2023 Jul 15;143:3-16. doi: 10.1016/j.semcdb.2022.03.022. Epub 2022 Mar 26. Semin Cell Dev Biol. 2023. PMID: 35351374 Review.
Cited by
-
Impact of aerobic exercise type on blood flow, muscle energy metabolism, and mitochondrial biogenesis in experimental lower extremity artery disease.Sci Rep. 2020 Aug 20;10(1):14048. doi: 10.1038/s41598-020-70961-8. Sci Rep. 2020. PMID: 32820213 Free PMC article.
-
The Effect of Diet on Improved Endurance in Male C57BL/6 Mice.Nutrients. 2018 Aug 16;10(8):1101. doi: 10.3390/nu10081101. Nutrients. 2018. PMID: 30115854 Free PMC article.
-
Exercise Training Protects against Atorvastatin-Induced Skeletal Muscle Dysfunction and Mitochondrial Dysfunction in the Skeletal Muscle of Rats.J Clin Med. 2020 Jul 19;9(7):2292. doi: 10.3390/jcm9072292. J Clin Med. 2020. PMID: 32707695 Free PMC article.
-
Early rehabilitation for volumetric muscle loss injury augments endogenous regenerative aspects of muscle strength and oxidative capacity.BMC Musculoskelet Disord. 2018 May 29;19(1):173. doi: 10.1186/s12891-018-2095-6. BMC Musculoskelet Disord. 2018. PMID: 29843673 Free PMC article.
-
What and How Can Physical Activity Prevention Function on Parkinson's Disease?Oxid Med Cell Longev. 2020 Feb 13;2020:4293071. doi: 10.1155/2020/4293071. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32215173 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

