Platelet-, monocyte-derived and tissue factor-carrying circulating microparticles are related to acute myocardial infarction severity
- PMID: 28207887
- PMCID: PMC5313202
- DOI: 10.1371/journal.pone.0172558
Platelet-, monocyte-derived and tissue factor-carrying circulating microparticles are related to acute myocardial infarction severity
Abstract
Objective: Circulating microparticles (cMPs) are phospholipid-rich vesicles released from cells when activated or injured, and contribute to the formation of intracoronary thrombi. Tissue factor (TF, CD142) is the main trigger of fibrin formation and TF-carrying cMPs are considered one of the most procoagulant cMPs. Similar types of atherosclerotic lesions may lead to different types of AMI, although the mechanisms behind are unresolved. Therefore, we aimed to investigate the phenotype of cMPs found in plasma of ACS patients and its relation to AMI severity and thrombotic burden.
Methods: In a cross-sectional study, two hundred patients aged 75±4 years were included in the study 2-8 weeks after suffering an AMI. Annexin V positive (AV+)-cMPs derived from blood and vascular cells were measured by flow cytometry. Plasma procoagulant activity (TF-PCA) was measured through a chromogenic assay.
Results: STEMI patients (n = 75) showed higher levels of platelet-derived cMPs [CD61+/AV+, CD31+/AV+, CD42b+/AV+ and CD31+/CD42b+/AV+, P = 0.048, 0.038, 0.009 and 0.006, respectively], compared to NSTEMI patients (n = 125). Patients who suffered a heart failure during AMI (n = 17) had increased levels of platelet (CD61+)-and monocyte (CD14+)-derived cMPs carrying TF (CD142+) (P<0.0001 and 0.004, respectively). Additionally, NYHA class III (n = 23) patients showed higher levels of CD142+/AV+, CD14+/AV+ and CD14+/CD142+/AV+ cMPs than those in class I/II (P = 0.001, 0.015 and 0.014, respectively). The levels of these cMPs positively correlated with TF-PCA (r≥0.166, P≤0.027, all).
Conclusions: Platelets and monocytes remain activated in AMI patients treated as per guidelines and release cMPs that discriminate AMI severity. Therefore, TF-MPs, and platelet- and monocyte-MPs may reflect thrombotic burden in AMI patients.
Conflict of interest statement
Figures


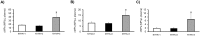
Similar articles
-
Monocyte-derived circulating microparticles (CD14+, CD14+/CD11b+ and CD14+/CD142+) are related to long-term prognosis for cardiovascular mortality in STEMI patients.Int J Cardiol. 2017 Jan 15;227:876-881. doi: 10.1016/j.ijcard.2016.11.302. Epub 2016 Nov 27. Int J Cardiol. 2017. PMID: 27915085
-
High levels of TSP1+/CD142+ platelet-derived microparticles characterise young patients with high cardiovascular risk and subclinical atherosclerosis.Thromb Haemost. 2015 Nov 25;114(6):1310-21. doi: 10.1160/TH15-04-0325. Epub 2015 Jul 16. Thromb Haemost. 2015. PMID: 26178021
-
Growing thrombi release increased levels of CD235a(+) microparticles and decreased levels of activated platelet-derived microparticles. Validation in ST-elevation myocardial infarction patients.J Thromb Haemost. 2015 Oct;13(10):1776-86. doi: 10.1111/jth.13065. Epub 2015 Sep 2. J Thromb Haemost. 2015. PMID: 26239059
-
Platelet microparticles and vascular cells interactions: a checkpoint between the haemostatic and thrombotic responses.Platelets. 2008 Feb;19(1):9-23. doi: 10.1080/09537100701817232. Platelets. 2008. PMID: 18231934 Review.
-
Exercise and Circulating Microparticles in Healthy Subjects.J Cardiovasc Transl Res. 2021 Oct;14(5):841-856. doi: 10.1007/s12265-021-10100-4. Epub 2021 Jan 25. J Cardiovasc Transl Res. 2021. PMID: 33495962 Review.
Cited by
-
Biology and Role of Extracellular Vesicles (EVs) in the Pathogenesis of Thrombosis.Int J Mol Sci. 2019 Jun 11;20(11):2840. doi: 10.3390/ijms20112840. Int J Mol Sci. 2019. PMID: 31212641 Free PMC article. Review.
-
Progress in extracellular vesicle homeostasis as it relates to cardiovascular diseases.J Physiol Biochem. 2024 Aug;80(3):511-522. doi: 10.1007/s13105-024-01027-w. Epub 2024 Apr 30. J Physiol Biochem. 2024. PMID: 38687443 Review.
-
Monocytes of Different Subsets in Complexes with Platelets in Patients with Myocardial Infarction.Thromb Haemost. 2018 Nov;118(11):1969-1981. doi: 10.1055/s-0038-1673342. Epub 2018 Oct 9. Thromb Haemost. 2018. PMID: 30300910 Free PMC article.
-
Changes in Circulating Extracellular Vesicles in Patients with ST-Elevation Myocardial Infarction and Potential Effects of Remote Ischemic Conditioning-A Randomized Controlled Trial.Biomedicines. 2020 Jul 16;8(7):218. doi: 10.3390/biomedicines8070218. Biomedicines. 2020. PMID: 32708657 Free PMC article.
-
Circulating Extracellular Vesicles As Biomarkers and Drug Delivery Vehicles in Cardiovascular Diseases.Biomolecules. 2021 Mar 5;11(3):388. doi: 10.3390/biom11030388. Biomolecules. 2021. PMID: 33808038 Free PMC article. Review.
References
-
- Chung SY, Tong MS, Sheu JJ, Lee FY, Sung PH, Chen CJ, et al. Short-term and long-term prognostic outcomes of patients with ST-segment elevation myocardial infarction complicated by profound cardiogenic shock undergoing early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention. Int J Cardiol. 2016;223:412–7. 10.1016/j.ijcard.2016.08.068 - DOI - PubMed
-
- Suades R, Padro T, Crespo J, Ramaiola I, Martin-Yuste V, Sabate M, et al. Circulating microparticle signature in coronary and peripheral blood of ST elevation myocardial infarction patients in relation to pain-to-PCI elapsed time. Int J Cardiol. 2016;202:378–87. 10.1016/j.ijcard.2015.09.011 - DOI - PubMed
-
- Suades R, Padro T, Vilahur G, Martin-Yuste V, Sabate M, Sans-Rosello J, et al. Growing thrombi release increased levels of CD235a(+) microparticles and decreased levels of activated platelet-derived microparticles. Validation in ST-elevation myocardial infarction patients. J Thromb Haemost. 2015;13(10):1776–86. 10.1111/jth.13065 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

