Effect of the different 13-valent pneumococcal conjugate vaccination uptakes on the invasive pneumococcal disease in children: Analysis of a hospital-based and population-based surveillance study in Madrid, Spain, 2007-2015
- PMID: 28207888
- PMCID: PMC5312951
- DOI: 10.1371/journal.pone.0172222
Effect of the different 13-valent pneumococcal conjugate vaccination uptakes on the invasive pneumococcal disease in children: Analysis of a hospital-based and population-based surveillance study in Madrid, Spain, 2007-2015
Abstract
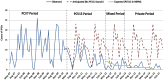
In the Community of Madrid, the 13-valent pneumococcal conjugate vaccine (PCV13) replaced the 7-valent (PCV7) in the fully government-funded Regional Immunization Program (RIP) in May, 2010, but was later excluded in May, 2012, and included again in January, 2015. These unique changes allowed us to assess the impact of the different pneumococcal vaccination policies on PCV13 uptake in infants and on the incidence rate (IR) of invasive pneumococcal disease (IPD) in children <15 years old. In this prospective, active, surveillance study, we estimated PCV13 uptakes, IR and incidence rate ratios (IRR) for total IPD and for IPD caused by PCV13- and non-PCV13 serotypes in children <15 years, stratified by age, in four periods with different vaccination policies: fully government-funded PCV7 vaccination, fully government-funded PCV13, mixed public/private funding and only private funding. Vaccine uptakes reached 95% in periods with public-funded pneumococcal vaccination, but fell to 67% in the private funding period. Overall, IR of IPD decreased by 68% (p<0.001) in 2014-15, due to 93% reduction in the IR of PCV13-type IPD (p<0.001) without significant changes in non-PCV13-type IPD. A fully government-funded PCV13 vaccination program lead to high vaccine uptake and dramatic reductions in both overall and PCV13-type IPD IR. When this program was switched to private PCV13 vaccination, there was a fall in vaccine coverage and stagnation in the decline of PCV13-type IPD with data suggesting a weakening of herd immunity.
Conflict of interest statement
Figures
Similar articles
-
Long-term Impact of a "3 + 0" Schedule for 7- and 13-Valent Pneumococcal Conjugate Vaccines on Invasive Pneumococcal Disease in Australia, 2002-2014.Clin Infect Dis. 2017 Jan 15;64(2):175-183. doi: 10.1093/cid/ciw720. Epub 2016 Oct 21. Clin Infect Dis. 2017. PMID: 27986682
-
Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway.Vaccine. 2013 Dec 16;31(52):6232-8. doi: 10.1016/j.vaccine.2013.10.032. Epub 2013 Oct 29. Vaccine. 2013. PMID: 24176490
-
Impact of pneumococcal conjugate vaccine (PCV7 and PCV13) on pneumococcal invasive diseases in Italian children and insight into evolution of pneumococcal population structure.Vaccine. 2017 Aug 16;35(35 Pt B):4587-4593. doi: 10.1016/j.vaccine.2017.07.010. Epub 2017 Jul 14. Vaccine. 2017. PMID: 28716556
-
Experience with pneumococcal polysaccharide conjugate vaccine (conjugated to CRM197 carrier protein) in children and adults.Clin Microbiol Infect. 2013 Oct;19 Suppl 1:1-9. doi: 10.1111/1469-0691.12320. Clin Microbiol Infect. 2013. PMID: 24083785 Review.
-
Pneumococcal serotype evolution in Western Europe.BMC Infect Dis. 2015 Oct 14;15:419. doi: 10.1186/s12879-015-1147-x. BMC Infect Dis. 2015. PMID: 26468008 Free PMC article. Review.
Cited by
-
Effectiveness of the 13-valent pneumococcal conjugate vaccine in preventing invasive pneumococcal disease in children aged 7-59 months. A matched case-control study.PLoS One. 2017 Aug 14;12(8):e0183191. doi: 10.1371/journal.pone.0183191. eCollection 2017. PLoS One. 2017. PMID: 28806737 Free PMC article.
-
Clinical and Economic Impact of a Potential Switch from 13-Valent to 10-Valent Pneumococcal Conjugate Infant Vaccination in Canada.Infect Dis Ther. 2018 Sep;7(3):353-371. doi: 10.1007/s40121-018-0206-1. Epub 2018 Jun 22. Infect Dis Ther. 2018. PMID: 29934878 Free PMC article.
-
Modeling the sustained use of the 13-valent pneumococcal conjugate vaccine compared to switching to the 10-valent vaccine in Mexico.Hum Vaccin Immunother. 2019;15(3):560-569. doi: 10.1080/21645515.2018.1516491. Epub 2018 Sep 21. Hum Vaccin Immunother. 2019. PMID: 30156978 Free PMC article.
-
Dominance of vaccine serotypes in pediatric invasive pneumococcal infections in Portugal (2012-2015).Sci Rep. 2019 Jan 9;9(1):6. doi: 10.1038/s41598-018-36799-x. Sci Rep. 2019. PMID: 30626918 Free PMC article.
-
Pneumococcal serotypes in children, clinical presentation and antimicrobial susceptibility in the PCV13 era.Epidemiol Infect. 2020 Nov 5;148:e279. doi: 10.1017/S0950268820002708. Epidemiol Infect. 2020. PMID: 33148361 Free PMC article.
References
-
- Aguiar SI, Brito MJ, Horacio AN, Lopes JP, Ramirez M, Melo-Cristino J; Portuguese Group for the Study of Streptococcal Infections; Portuguese Study Group of Invasive Pneumococcal Disease of the Paediatric Infectious Disease Society. Decreasing incidence and changes in serotype distribution of invasive pneumococcal disease in persons aged under 18 years since introduction of 10-valent and 13-valent conjugate vaccines in Portugal, July 2008 to June 2012. Euro Surveill 2014; 19: 20750 - PubMed
-
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15: 301–309. 10.1016/S1473-3099(14)71081-3 - DOI - PMC - PubMed
-
- Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis 2015; 15: 535–543. 10.1016/S1473-3099(15)70044-7 - DOI - PubMed
-
- Centers for Disease Control and Prevention. 1998. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 1998. http://www.cdc.gov/abcs/reports-findings/survreports/spneu98.pdf
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

