Optimization of intestinal microsomal preparation in the rat: A systematic approach to assess the influence of various methodologies on metabolic activity and scaling factors
- PMID: 28207929
- PMCID: PMC5413848
- DOI: 10.1002/bdd.2070
Optimization of intestinal microsomal preparation in the rat: A systematic approach to assess the influence of various methodologies on metabolic activity and scaling factors
Abstract
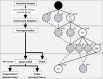
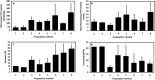
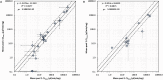
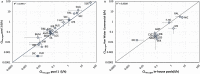
The metabolic capacity of the intestine and its importance as the initial barrier to systemic exposure can lead to underestimation of first-pass, and thus overestimation of oral bioavailability. However, the in vitro tools informing estimates of in vivo intestinal metabolism are limited by the complexity of the in vitro matrix preparation and uncertainty with the scaling factors for in vitro to in vivo extrapolation. A number of methods currently exist in the literature for the preparation of intestinal microsomes; however, the impact of key steps in the preparation procedure has not been critically assessed. In the current study, changes in enterocyte isolation, the impact of buffer constituents heparin and glycerol, as well as sonication as a direct method of homogenization were assessed systematically. Furthermore, fresh vs. frozen tissue samples and the impact of microsome freeze thawing was assessed. The rat intestinal microsomes were characterized for CYP content as well as metabolic activity using testosterone and 4-nitropheonol as probes for CYP and UGT activity, respectively. Comparisons in metabolic activity and scaled unbound intestinal intrinsic clearance (CLintu,gut ) were made to commercially available microsomes using 25 drugs with a diverse range of metabolic pathways and intestinal metabolic stabilities. An optimal, robust and reproducible microsomal preparation method for investigation of intestinal metabolism is proposed. The importance of characterization of the in vitro matrix and the potential impact of intestinal scaling factors on the in vitro-in vivo extrapolation of FG needs to be investigated further. © 2017 The Authors Biopharmaceutics & Drug Disposition Published by John Wiley & Sons Ltd.
Keywords: in vitro-in vivo extrapolation; intestinal metabolism; scaling factors.
© 2017 The Authors Biopharmaceutics & Drug Disposition Published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Catalytic activities of cytochrome P450 enzymes and UDP-glucuronosyltransferases involved in drug metabolism in rat everted sacs and intestinal microsomes.Xenobiotica. 2003 Jan;33(1):43-55. doi: 10.1080/0049825021000022348. Xenobiotica. 2003. PMID: 12519693
-
Prediction of hepatic and intestinal glucuronidation using in vitro-in vivo extrapolation.Drug Metab Pharmacokinet. 2015 Feb;30(1):21-9. doi: 10.1016/j.dmpk.2014.10.001. Epub 2014 Oct 13. Drug Metab Pharmacokinet. 2015. PMID: 25760528 Review.
-
Prediction of human drug clearance by multiple metabolic pathways: integration of hepatic and intestinal microsomal and cytosolic data.Drug Metab Dispos. 2011 May;39(5):864-73. doi: 10.1124/dmd.110.036566. Epub 2011 Feb 8. Drug Metab Dispos. 2011. PMID: 21303923
-
Development of an optimized procedure for the preparation of rat intestinal microsomes: comparison of hepatic and intestinal microsomal cytochrome P450 enzyme activities in two rat strains.Xenobiotica. 2009 Jan;39(1):22-32. doi: 10.1080/00498250802517714. Xenobiotica. 2009. PMID: 19219745
-
Challenges and Opportunities with Non-CYP Enzymes Aldehyde Oxidase, Carboxylesterase, and UDP-Glucuronosyltransferase: Focus on Reaction Phenotyping and Prediction of Human Clearance.AAPS J. 2016 Nov;18(6):1391-1405. doi: 10.1208/s12248-016-9962-6. Epub 2016 Aug 5. AAPS J. 2016. PMID: 27495117 Free PMC article. Review.
Cited by
-
Quantifying gut wall metabolism: methodology matters.Biopharm Drug Dispos. 2017 Mar;38(2):155-160. doi: 10.1002/bdd.2062. Epub 2017 Feb 14. Biopharm Drug Dispos. 2017. PMID: 28039878 Free PMC article. No abstract available.
-
Parameterization of Microsomal and Cytosolic Scaling Factors: Methodological and Biological Considerations for Scalar Derivation and Validation.Eur J Drug Metab Pharmacokinet. 2021 Mar;46(2):173-183. doi: 10.1007/s13318-020-00666-w. Epub 2020 Dec 19. Eur J Drug Metab Pharmacokinet. 2021. PMID: 33340340
-
Microsomal and Cytosolic Scaling Factors in Dog and Human Kidney Cortex and Application for In Vitro-In Vivo Extrapolation of Renal Metabolic Clearance.Drug Metab Dispos. 2017 May;45(5):556-568. doi: 10.1124/dmd.117.075242. Epub 2017 Mar 7. Drug Metab Dispos. 2017. PMID: 28270564 Free PMC article.
-
Effects of aqueous extract from Baiyedancong-Oolong tea on cytochrome P450 enzymes activities, P-gp and OATs transport abilities and transcription levels in mice.Front Nutr. 2023 May 9;10:1136329. doi: 10.3389/fnut.2023.1136329. eCollection 2023. Front Nutr. 2023. PMID: 37229476 Free PMC article.
-
The impact of freeze-dried Baiyedancong-Oolong tea aqueous extract containing bioactive compounds on the activities of CYP450 enzymes, the transport capabilities of P-gp and OATs, and transcription levels in mice.Food Nutr Res. 2024 Sep 27;68. doi: 10.29219/fnr.v68.10605. eCollection 2024. Food Nutr Res. 2024. PMID: 39376904 Free PMC article.
References
-
- Pond SM, Tozer TN. First‐pass elimination. Basic concepts and clinical consequences. Clin Pharmacokinet 1984; 9: 1–25. - PubMed
-
- van de Waterbeemd H, Gifford E. ADMET in silico modelling: towards prediction paradise? Nat Rev Drug Discov 2003; 2: 192–204. - PubMed
-
- Lin JH, Chiba M, Baillie TA. Is the role of the small intestine in first‐pass metabolism overemphasized? Pharmacol Rev 1999; 51: 135–158. - PubMed
-
- Gertz M, Davis JD, Harrison A, Houston JB, Galetin A. Grapefruit juice–drug interaction studies as a method to assess the extent of intestinal availability: utility and limitations. Curr Drug Metab 2008a; 9: 785–795. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

