Optimization of intestinal microsomal preparation in the rat: A systematic approach to assess the influence of various methodologies on metabolic activity and scaling factors
- PMID: 28207929
- PMCID: PMC5413848
- DOI: 10.1002/bdd.2070
Optimization of intestinal microsomal preparation in the rat: A systematic approach to assess the influence of various methodologies on metabolic activity and scaling factors
Abstract
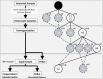
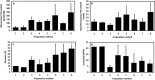
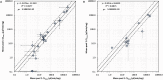
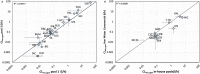
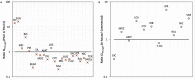
The metabolic capacity of the intestine and its importance as the initial barrier to systemic exposure can lead to underestimation of first-pass, and thus overestimation of oral bioavailability. However, the in vitro tools informing estimates of in vivo intestinal metabolism are limited by the complexity of the in vitro matrix preparation and uncertainty with the scaling factors for in vitro to in vivo extrapolation. A number of methods currently exist in the literature for the preparation of intestinal microsomes; however, the impact of key steps in the preparation procedure has not been critically assessed. In the current study, changes in enterocyte isolation, the impact of buffer constituents heparin and glycerol, as well as sonication as a direct method of homogenization were assessed systematically. Furthermore, fresh vs. frozen tissue samples and the impact of microsome freeze thawing was assessed. The rat intestinal microsomes were characterized for CYP content as well as metabolic activity using testosterone and 4-nitropheonol as probes for CYP and UGT activity, respectively. Comparisons in metabolic activity and scaled unbound intestinal intrinsic clearance (CLintu,gut ) were made to commercially available microsomes using 25 drugs with a diverse range of metabolic pathways and intestinal metabolic stabilities. An optimal, robust and reproducible microsomal preparation method for investigation of intestinal metabolism is proposed. The importance of characterization of the in vitro matrix and the potential impact of intestinal scaling factors on the in vitro-in vivo extrapolation of FG needs to be investigated further. © 2017 The Authors Biopharmaceutics & Drug Disposition Published by John Wiley & Sons Ltd.
Keywords: in vitro-in vivo extrapolation; intestinal metabolism; scaling factors.
© 2017 The Authors Biopharmaceutics & Drug Disposition Published by John Wiley & Sons Ltd.
Figures
References
-
- Pond SM, Tozer TN. First‐pass elimination. Basic concepts and clinical consequences. Clin Pharmacokinet 1984; 9: 1–25. - PubMed
-
- van de Waterbeemd H, Gifford E. ADMET in silico modelling: towards prediction paradise? Nat Rev Drug Discov 2003; 2: 192–204. - PubMed
-
- Lin JH, Chiba M, Baillie TA. Is the role of the small intestine in first‐pass metabolism overemphasized? Pharmacol Rev 1999; 51: 135–158. - PubMed
-
- Gertz M, Davis JD, Harrison A, Houston JB, Galetin A. Grapefruit juice–drug interaction studies as a method to assess the extent of intestinal availability: utility and limitations. Curr Drug Metab 2008a; 9: 785–795. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

