Feasibility Assessment of Patient Reporting of Symptomatic Adverse Events in Multicenter Cancer Clinical Trials
- PMID: 28208174
- PMCID: PMC5553624
- DOI: 10.1001/jamaoncol.2016.6749
Feasibility Assessment of Patient Reporting of Symptomatic Adverse Events in Multicenter Cancer Clinical Trials
Abstract
Importance: In cancer clinical trials, symptomatic adverse events (AEs), such as nausea, are reported by investigators rather than by patients. There is increasing interest to collect symptomatic AE data via patient-reported outcome (PRO) questionnaires, but it is unclear whether it is feasible to implement this approach in multicenter trials.
Objective: To examine whether patients are willing and able to report their symptomatic AEs in multicenter trials.
Design, setting, and participants: A total of 361 consecutive patients enrolled in any 1 of 9 US multicenter cancer treatment trials were invited to self-report 13 common symptomatic AEs using a PRO adaptation of the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) via tablet computers at 5 successive clinic visits. Patient adherence was tracked with reasons for missed self-reports. Agreement with clinician AE reports was analyzed with weighted κ statistics. Patient and investigator perspectives were elicited by survey. The study was conducted from March 15, 2007, to August 11, 2011. Data analysis was performed from August 9, 2013, to March 21, 2014.
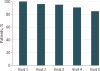
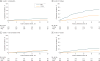
Results: Of the 361 patients invited to participate, 285 individuals enrolled, with a median age of 57 years (range, 24-88), 202 (74.3%) female, 241 (85.5%) white, 73 (26.8%) with a high school education or less, and 176 (64.7%) who reported regular internet use (denominators varied owing to missing data). Across all patients and trials, there were 1280 visits during which patients had an opportunity to self-report (ie, patients were alive and enrolled in a treatment trial at the time of the visit). Self-reports were completed at 1202 visits (93.9% overall adherence). Adherence was highest at baseline and declined over time (visit 1, 100%; visit 2, 96%; visit 3, 95%; visit 4, 91%; and visit 5, 85%). Reasons for missing PROs included institutional errors in 27 of 48 (56.3%) of the cases (eg, staff forgetting to bring computers to patients at visits), patients feeling "too ill" in 8 (16.7%), patient refusal in 8 (16.7%), and internet connectivity problems in 5 (10.4%). Patient-investigator CTCAE agreement was moderate or worse for most symptoms (most κ < 0.05), with investigators reporting fewer AEs than patients across symptoms. Most patients believed that the system was easy to use (234 [93.2%]) and useful (230 [93.1%]), and investigators thought that the patient-reported AEs were useful (133 [94.3%]) and accurate (119 [83.2%]).
Conclusions and relevance: Participants in multicenter cancer trials are willing and able to report their own symptomatic AEs at most clinic visits and report more AEs than investigators. This approach may improve the precision of AE reporting in cancer trials.
Conflict of interest statement
Figures
Comment in
-
Improving the Evidence Base for Delivery of High-Quality Cancer Care.JAMA Oncol. 2017 Aug 1;3(8):1029-1031. doi: 10.1001/jamaoncol.2016.6722. JAMA Oncol. 2017. PMID: 28208180 No abstract available.
Similar articles
-
Feasibility of Patient Reporting of Symptomatic Adverse Events via the Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) in a Chemoradiotherapy Cooperative Group Multicenter Clinical Trial.Int J Radiat Oncol Biol Phys. 2017 Jun 1;98(2):409-418. doi: 10.1016/j.ijrobp.2017.02.002. Epub 2017 Feb 10. Int J Radiat Oncol Biol Phys. 2017. PMID: 28463161 Free PMC article. Clinical Trial.
-
Patient free text reporting of symptomatic adverse events in cancer clinical research using the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).J Am Med Inform Assoc. 2019 Apr 1;26(4):276-285. doi: 10.1093/jamia/ocy169. J Am Med Inform Assoc. 2019. PMID: 30840079 Free PMC article.
-
Assessment of Adverse Events From the Patient Perspective in a Phase 3 Metastatic Castration-Resistant Prostate Cancer Clinical Trial.JAMA Oncol. 2020 Feb 1;6(2):e193332. doi: 10.1001/jamaoncol.2019.3332. Epub 2020 Feb 13. JAMA Oncol. 2020. PMID: 31556911 Free PMC article. Clinical Trial.
-
Patient-Reported Outcomes in Cancer Clinical Trials: Measuring Symptomatic Adverse Events With the National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).Am Soc Clin Oncol Educ Book. 2016;35:67-73. doi: 10.1200/EDBK_159514. Am Soc Clin Oncol Educ Book. 2016. PMID: 27249687 Review.
-
Supporting quality and patient safety in cancer clinical trials.Clin J Oncol Nurs. 2011 Jun;15(3):263-5. doi: 10.1188/11.CJON.263-265. Clin J Oncol Nurs. 2011. PMID: 21624861 Review.
Cited by
-
Digital Patient-Reported Outcome Measures Assessing Health-Related Quality of Life in Skull Base Diseases-Analysis of Feasibility and Pitfalls Two Years after Implementation.Healthcare (Basel). 2023 Feb 6;11(4):472. doi: 10.3390/healthcare11040472. Healthcare (Basel). 2023. PMID: 36833006 Free PMC article.
-
Longitudinal Patient Reported Outcomes with CAR-T Cell Therapy Versus Autologous and Allogeneic Stem Cell Transplant.Transplant Cell Ther. 2022 Aug;28(8):473-482. doi: 10.1016/j.jtct.2022.05.004. Epub 2022 May 9. Transplant Cell Ther. 2022. PMID: 35550440 Free PMC article.
-
Validation study of the Japanese version of MD Anderson Symptom Inventory for Brain Tumor module.Jpn J Clin Oncol. 2020 Jul 9;50(7):787-793. doi: 10.1093/jjco/hyaa036. Jpn J Clin Oncol. 2020. PMID: 32280995 Free PMC article.
-
Completion of Patient-Reported Outcome Questionnaires Among Older Adults with Advanced Cancer.J Pain Symptom Manage. 2022 Feb;63(2):301-310. doi: 10.1016/j.jpainsymman.2021.07.032. Epub 2021 Aug 8. J Pain Symptom Manage. 2022. PMID: 34371137 Free PMC article. Clinical Trial.
-
Results from a 1-day workshop on the assessment of quality of life in cancer patients: a joint initiative of the Japan Clinical Oncology Group and the European Organisation for Research and Treatment of Cancer.Jpn J Clin Oncol. 2020 Oct 22;50(11):1333-1341. doi: 10.1093/jjco/hyaa119. Jpn J Clin Oncol. 2020. PMID: 32783053 Free PMC article.
References
-
- National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), version 3. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/.... Published August 9, 2006. Accessed March 23, 2016.
-
- Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25(32):5121–5127. - PubMed
-
- Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How accurate is clinician reporting of chemotherapy adverse effects? a comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22(17):3485–3490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA077658/CA/NCI NIH HHS/United States
- U10 CA086726/CA/NCI NIH HHS/United States
- U10 CA035279/CA/NCI NIH HHS/United States
- U10 CA035421/CA/NCI NIH HHS/United States
- U10 CA114558/CA/NCI NIH HHS/United States
- U10 CA045418/CA/NCI NIH HHS/United States
- UG1 CA189817/CA/NCI NIH HHS/United States
- UG1 CA189823/CA/NCI NIH HHS/United States
- UG1 CA189972/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- UG1 CA189853/CA/NCI NIH HHS/United States
- UG1 CA189819/CA/NCI NIH HHS/United States
- P30 CA016359/CA/NCI NIH HHS/United States
- U10 CA047642/CA/NCI NIH HHS/United States
- U10 CA138561/CA/NCI NIH HHS/United States
- UG1 CA189829/CA/NCI NIH HHS/United States
- U10 CA032291/CA/NCI NIH HHS/United States
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA045389/CA/NCI NIH HHS/United States
- U10 CA180854/CA/NCI NIH HHS/United States
- U10 CA021060/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- U10 CA180836/CA/NCI NIH HHS/United States
- UG1 CA189858/CA/NCI NIH HHS/United States
- U10 CA180791/CA/NCI NIH HHS/United States
- U10 CA037447/CA/NCI NIH HHS/United States
- U10 CA180850/CA/NCI NIH HHS/United States
- UG1 CA189850/CA/NCI NIH HHS/United States
- UG1 CA189830/CA/NCI NIH HHS/United States
- U10 CA041287/CA/NCI NIH HHS/United States
- U10 CA047559/CA/NCI NIH HHS/United States
- U10 CA077651/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180838/CA/NCI NIH HHS/United States
- U10 CA180844/CA/NCI NIH HHS/United States
- U10 CA003927/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

