The Role of Nerve Growth Factor (NGF) and Its Precursor Forms in Oral Wound Healing
- PMID: 28208669
- PMCID: PMC5343921
- DOI: 10.3390/ijms18020386
The Role of Nerve Growth Factor (NGF) and Its Precursor Forms in Oral Wound Healing
Abstract
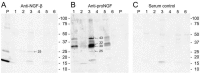
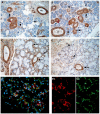
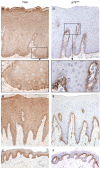
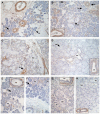
Nerve growth factor (NGF) and its different precursor forms are secreted into human saliva by salivary glands and are also produced by an array of cells in the tissues of the oral cavity. The major forms of NGF in human saliva are forms of pro-nerve growth factor (pro-NGF) and not mature NGF. The NGF receptors tropomyosin-related kinase A (TrkA) and p75 neurotrophin receptor (p75NTR) are widely expressed on cells in the soft tissues of the human oral cavity, including keratinocytes, endothelial cells, fibroblasts and leukocytes, and in ductal and acinar cells of all types of salivary glands. In vitro models show that NGF can contribute at most stages in the oral wound healing process: restitution, cell survival, apoptosis, cellular proliferation, inflammation, angiogenesis and tissue remodeling. NGF may therefore take part in the effective wound healing in the oral cavity that occurs with little scarring. As pro-NGF forms appear to be the major form of NGF in human saliva, efforts should be made to study its function, specifically in the process of wound healing. In addition, animal and clinical studies should be initiated to examine if topical application of pro-NGF or NGF can be a therapy for chronic oral ulcerations and wounds.
Keywords: keratinocytes; oral cavity; saliva; salivary gland.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Distribution of nerve growth factor, pro-nerve growth factor, and their receptors in human salivary glands.Eur J Oral Sci. 2013 Feb;121(1):13-20. doi: 10.1111/eos.12008. Epub 2012 Dec 14. Eur J Oral Sci. 2013. PMID: 23331419
-
Nerve growth factor beta/pro-nerve growth factor and their receptors in normal human oral mucosa.Eur J Oral Sci. 2007 Oct;115(5):344-54. doi: 10.1111/j.1600-0722.2007.00480.x. Eur J Oral Sci. 2007. PMID: 17850422
-
Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice.Diabetologia. 2004 Jun;47(6):1047-54. doi: 10.1007/s00125-004-1414-7. Epub 2004 May 26. Diabetologia. 2004. PMID: 15164170
-
Nerve growth factor and tissue repair remodeling: trkA(NGFR) and p75(NTR), two receptors one fate.Cytokine Growth Factor Rev. 2007 Jun-Aug;18(3-4):245-56. doi: 10.1016/j.cytogfr.2007.04.004. Epub 2007 May 24. Cytokine Growth Factor Rev. 2007. PMID: 17531524 Review.
-
Nerve growth factor and burn wound healing: Update of molecular interactions with skin cells.Burns. 2023 Aug;49(5):989-1002. doi: 10.1016/j.burns.2022.11.001. Epub 2022 Nov 7. Burns. 2023. PMID: 36379825 Review.
Cited by
-
Updating on the Dual Role of Salivary Gland Epithelial Cell (SGEC) in Sjögren's Disease.J Inflamm Res. 2025 Mar 1;18:3039-3053. doi: 10.2147/JIR.S509220. eCollection 2025. J Inflamm Res. 2025. PMID: 40046682 Free PMC article. Review.
-
All-in-one smart dressing for simultaneous angiogenesis and neural regeneration.J Nanobiotechnology. 2023 Feb 3;21(1):38. doi: 10.1186/s12951-023-01787-5. J Nanobiotechnology. 2023. PMID: 36737778 Free PMC article.
-
Novel impacts of saliva with regard to oral health.J Prosthet Dent. 2022 Mar;127(3):383-391. doi: 10.1016/j.prosdent.2021.05.009. Epub 2021 Jun 16. J Prosthet Dent. 2022. PMID: 34140141 Free PMC article. Review.
-
[Advance of new dressings for promoting skin wound healing].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2019 Dec 25;36(6):1055-1059. doi: 10.7507/1001-5515.201811023. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2019. PMID: 31875383 Free PMC article. Review. Chinese.
-
Anthocyanins Extracted from Oryza sativa L. Prevent Fluorouracil-Induced Nuclear Factor-κB Activation in Oral Mucositis: In Vitro and In Vivo Studies.Int J Mol Sci. 2018 Sep 29;19(10):2981. doi: 10.3390/ijms19102981. Int J Mol Sci. 2018. PMID: 30274314 Free PMC article.
References
-
- Fahnestock M. Structure and biosynthesis of nerve growth factor. Curr. Top. Microbiol. Immunol. 1991;165:1–26. - PubMed
-
- Lipps B.V. Isolation of nerve growth factor (NGF) from human body fluids; saliva, serum and urine: Comparison between cobra venom and cobra serum NGF. J. Nat. Toxins. 2000;9:349–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

