The Glutamate Dehydrogenase Pathway and Its Roles in Cell and Tissue Biology in Health and Disease
- PMID: 28208702
- PMCID: PMC5372004
- DOI: 10.3390/biology6010011
The Glutamate Dehydrogenase Pathway and Its Roles in Cell and Tissue Biology in Health and Disease
Abstract
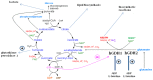
Glutamate dehydrogenase (GDH) is a hexameric enzyme that catalyzes the reversible conversion of glutamate to α-ketoglutarate and ammonia while reducing NAD(P)⁺ to NAD(P)H. It is found in all living organisms serving both catabolic and anabolic reactions. In mammalian tissues, oxidative deamination of glutamate via GDH generates α-ketoglutarate, which is metabolized by the Krebs cycle, leading to the synthesis of ATP. In addition, the GDH pathway is linked to diverse cellular processes, including ammonia metabolism, acid-base equilibrium, redox homeostasis (via formation of fumarate), lipid biosynthesis (via oxidative generation of citrate), and lactate production. While most mammals possess a single GDH1 protein (hGDH1 in the human) that is highly expressed in the liver, humans and other primates have acquired, via duplication, an hGDH2 isoenzyme with distinct functional properties and tissue expression profile. The novel hGDH2 underwent rapid evolutionary adaptation, acquiring unique properties that enable enhanced enzyme function under conditions inhibitory to its ancestor hGDH1. These are thought to provide a biological advantage to humans with hGDH2 evolution occurring concomitantly with human brain development. hGDH2 is co-expressed with hGDH1 in human brain, kidney, testis and steroidogenic organs, but not in the liver. In human cerebral cortex, hGDH1 and hGDH2 are expressed in astrocytes, the cells responsible for removing and metabolizing transmitter glutamate, and for supplying neurons with glutamine and lactate. In human testis, hGDH2 (but not hGDH1) is densely expressed in the Sertoli cells, known to provide the spermatids with lactate and other nutrients. In steroid producing cells, hGDH1/2 is thought to generate reducing equivalents (NADPH) in the mitochondria for the biosynthesis of steroidal hormones. Lastly, up-regulation of hGDH1/2 expression occurs in cancer, permitting neoplastic cells to utilize glutamine/glutamate for their growth. In addition, deregulation of hGDH1/2 is implicated in the pathogenesis of several human disorders.
Keywords: GDH; GDH deregulation and diseases; expression; glioma; hGDH1; hGDH2; human tissues; regulation; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Smith E.L., Austin B.M., Blumenthal K.M., Nyc J.F. In: The Enzymes. Boyer P.D., editor. Academic Press; New York, NY, USA: 1975. pp. 293–367.
-
- Jin L., Li D., Alesi G.N., Fan J., Kang H.B., Lu Z., Boggon T.J., Jin P., Yi H., Wright E.R., et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell. 2015;27:257–270. doi: 10.1016/j.ccell.2014.12.006. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

