Splicing and Polyadenylation of Human Papillomavirus Type 16 mRNAs
- PMID: 28208770
- PMCID: PMC5343901
- DOI: 10.3390/ijms18020366
Splicing and Polyadenylation of Human Papillomavirus Type 16 mRNAs
Abstract
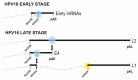
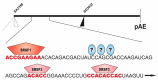
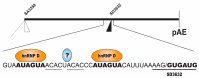
The human papillomavirus type 16 (HPV16) life cycle can be divided into an early stage in which the HPV16 genomic DNA is replicated, and a late stage in which the HPV16 structural proteins are synthesized and virions are produced. A strong coupling between the viral life cycle and the differentiation state of the infected cell is highly characteristic of all HPVs. The switch from the HPV16 early gene expression program to the late requires a promoter switch, a polyadenylation signal switch and a shift in alternative splicing. A number of cis-acting RNA elements on the HPV16 mRNAs and cellular and viral factors interacting with these elements are involved in the control of HPV16 gene expression. This review summarizes our knowledge of HPV16 cis-acting RNA elements and cellular and viral trans-acting factors that regulate HPV16 gene expression at the level of splicing and polyadenylation.
Keywords: HPV16; SR-protein; hnRNP; human papillomavirus; polyadenylation; splicing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

