The Therapeutic Potential of Migrastatin-Core Analogs for the Treatment of Metastatic Cancer
- PMID: 28208778
- PMCID: PMC6155687
- DOI: 10.3390/molecules22020198
The Therapeutic Potential of Migrastatin-Core Analogs for the Treatment of Metastatic Cancer
Abstract
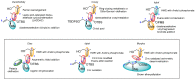
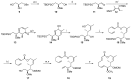
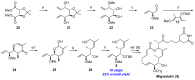
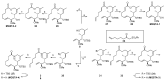
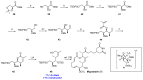
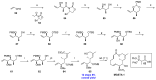
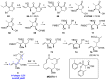
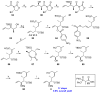
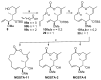
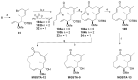
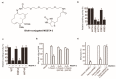
Tumor metastasis is a complex process in which cells detach from the primary tumor and colonize a distant organ. Metastasis is also the main process responsible for cancer-related death. Despite the enormous efforts made to unravel the metastatic process, there is no effective therapy, and patients with metastatic tumors have poor prognosis. In this regard, there is an urgent need for new therapeutic tools for the treatment of this disease. Small molecules with the capacity to reduce cell migration could be used to treat metastasis. Migrastatin-core analogs are naturally inspired macrocycles that inhibit pathological cell migration and are able to reduce metastasis in animal models. Migrastatin analogs can be synthesized from a common advanced intermediate. Herein we present a review of the synthetic approaches that can be used to prepare this key intermediate, together with a review of the biological activity of migrastatin-core analogs and current hypotheses concerning their mechanism of action.
Keywords: cancer metastasis; diverted total synthesis; natural products.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








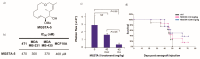

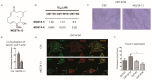
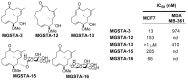
Similar articles
-
Diverted total synthesis leads to the generation of promising cell-migration inhibitors for treatment of tumor metastasis: in vivo and mechanistic studies on the migrastatin core ether analog.J Am Chem Soc. 2010 Mar 10;132(9):3224-8. doi: 10.1021/ja9101503. J Am Chem Soc. 2010. PMID: 20155906 Free PMC article.
-
Migrastatin analogues target fascin to block tumour metastasis.Nature. 2010 Apr 15;464(7291):1062-6. doi: 10.1038/nature08978. Nature. 2010. PMID: 20393565 Free PMC article.
-
Quantitative structure-activity studies on a series of migrastatin analogs as inhibitors of cancer cell metastasis.Med Chem. 2011 May;7(3):155-64. doi: 10.2174/157340611795564240. Med Chem. 2011. PMID: 21486209
-
On the potential of natural products in the discovery of pharma leads: a case for reassessment.Nat Prod Rep. 2010 Aug;27(8):1114-6. doi: 10.1039/c003211p. Epub 2010 Apr 9. Nat Prod Rep. 2010. PMID: 20383353 Review.
-
Natural products as anti-invasive and anti-metastatic agents.Curr Med Chem. 2011;18(6):808-29. doi: 10.2174/092986711794927711. Curr Med Chem. 2011. PMID: 21182481 Review.
Cited by
-
Bio-Guided Isolation of Antimalarial Metabolites from the Coculture of Two Red Sea Sponge-Derived Actinokineospora and Rhodococcus spp.Mar Drugs. 2021 Feb 12;19(2):109. doi: 10.3390/md19020109. Mar Drugs. 2021. PMID: 33673168 Free PMC article.
-
Knockout of glucosidase II beta subunit inhibits growth and metastatic potential of lung cancer cells by inhibiting receptor tyrosine kinase activities.Sci Rep. 2019 Jul 17;9(1):10394. doi: 10.1038/s41598-019-46701-y. Sci Rep. 2019. PMID: 31316108 Free PMC article.
References
-
- Nakae K., Yoshimoto Y., Sawa T., Homma Y., Hamada M., Takeuchi T., Imoto M. Migrastatin, a new inhibitor of tumor cell migration from Streptomyces sp. MK929-43F1. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 2000;53:1130–1136. doi: 10.7164/antibiotics.53.1130. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

