Gene regulatory network underlying the immortalization of epithelial cells
- PMID: 28209158
- PMCID: PMC5314717
- DOI: 10.1186/s12918-017-0393-5
Gene regulatory network underlying the immortalization of epithelial cells
Abstract
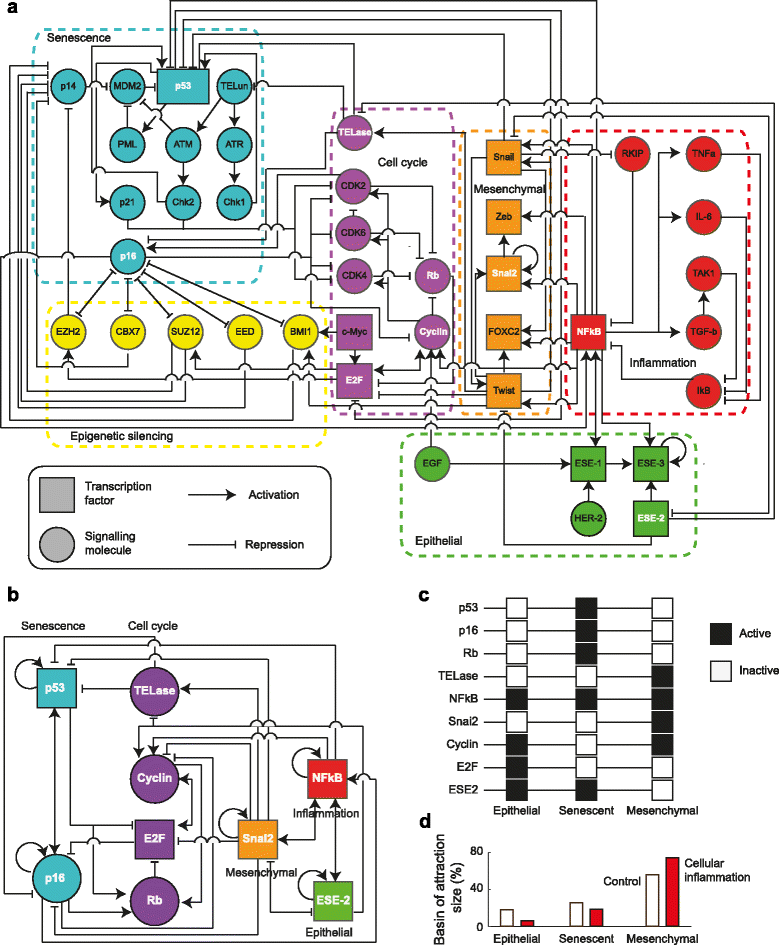
Background: Tumorigenic transformation of human epithelial cells in vitro has been described experimentally as the potential result of spontaneous immortalization. This process is characterized by a series of cell-state transitions, in which normal epithelial cells acquire first a senescent state which is later surpassed to attain a mesenchymal stem-like phenotype with a potentially tumorigenic behavior. In this paper we aim to provide a system-level mechanistic explanation to the emergence of these cell types, and to the time-ordered transition patterns that are common to neoplasias of epithelial origin. To this end, we first integrate published functional and well-curated molecular data of the components and interactions that have been found to be involved in such cell states and transitions into a network of 41 molecular components. We then reduce this initial network by removing simple mediators (i.e., linear pathways), and formalize the resulting regulatory core into logical rules that govern the dynamics of each of the network components as a function of the states of its regulators.
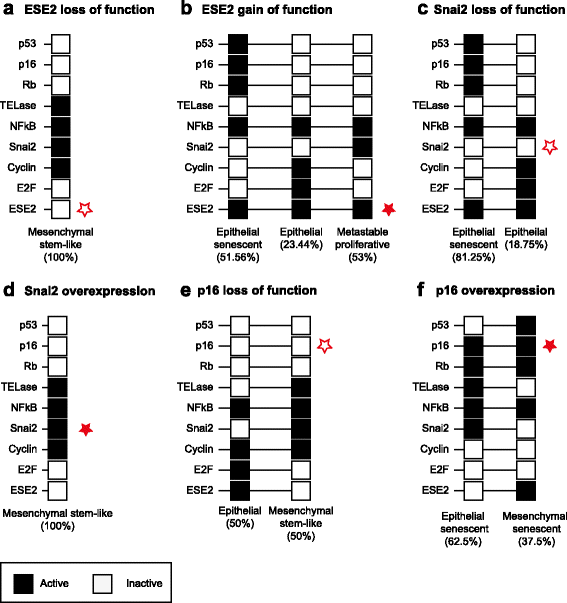
Results: Computational dynamic analysis shows that our proposed Gene Regulatory Network model recovers exactly three attractors, each of them defined by a specific gene expression profile that corresponds to the epithelial, senescent, and mesenchymal stem-like cellular phenotypes, respectively. We show that although a mesenchymal stem-like state can be attained even under unperturbed physiological conditions, the likelihood of converging to this state is increased when pro-inflammatory conditions are simulated, providing a systems-level mechanistic explanation for the carcinogenic role of chronic inflammatory conditions observed in the clinic. We also found that the regulatory core yields an epigenetic landscape that restricts temporal patterns of progression between the steady states, such that recovered patterns resemble the time-ordered transitions observed during the spontaneous immortalization of epithelial cells, both in vivo and in vitro.
Conclusion: Our study strongly suggests that the in vitro tumorigenic transformation of epithelial cells, which strongly correlates with the patterns observed during the pathological progression of epithelial carcinogenesis in vivo, emerges from underlying regulatory networks involved in epithelial trans-differentiation during development.
Keywords: Boolean models; Carcinomas; Epigenetic landscape; Gene regulatory networks; Phenotypic attractors.
Figures

References
-
- Huang S. On the intrinsic inevitability of cancer: from foetal to fatal attraction. In: Seminars in cancer biology. vol. 21, No. 3. Academic Press: 2011. p. 183–99. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

