Rethinking the role of alpha toxin in Clostridium perfringens-associated enteric diseases: a review on bovine necro-haemorrhagic enteritis
- PMID: 28209206
- PMCID: PMC5314468
- DOI: 10.1186/s13567-017-0413-x
Rethinking the role of alpha toxin in Clostridium perfringens-associated enteric diseases: a review on bovine necro-haemorrhagic enteritis
Abstract
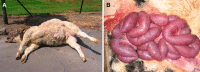
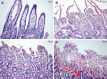
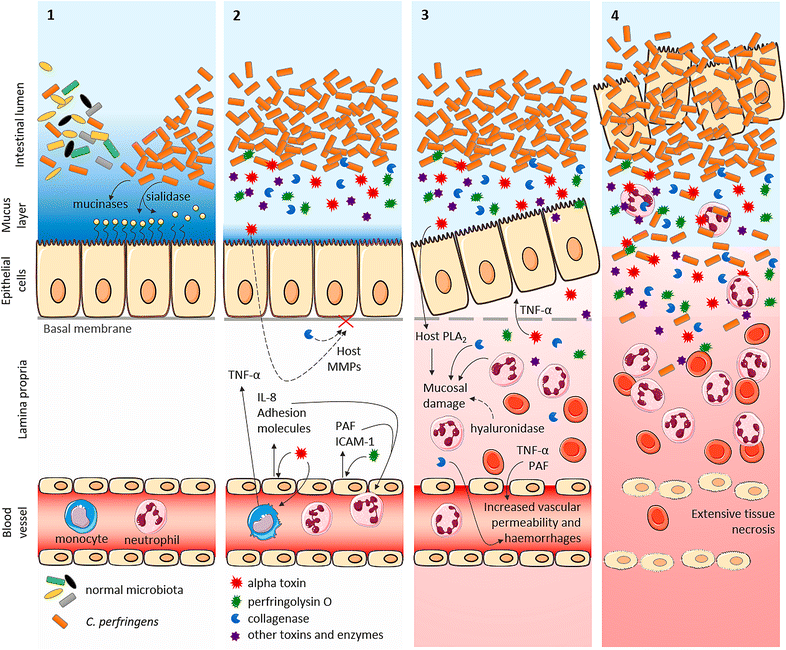
Bovine necro-haemorrhagic enteritis is an economically important disease caused by Clostridium perfringens type A strains. The disease mainly affects calves under intensive rearing conditions and is characterized by sudden death associated with small intestinal haemorrhage, necrosis and mucosal neutrophil infiltration. The common assumption that, when causing intestinal disease, C. perfringens relies upon specific, plasmid-encoded toxins, was recently challenged by the finding that alpha toxin, which is produced by all C. perfringens strains, is essential for necro-haemorrhagic enteritis. In addition to alpha toxin, other C. perfringens toxins and/or enzymes might contribute to the pathogenesis of necro-haemorrhagic enteritis. These additional virulence factors might contribute to breakdown of the protective mucus layer during initial stage of pathogenesis, after which alpha toxin, either or not in synergy with other toxins such as perfringolysin O, can act on the mucosal tissue. Furthermore, alpha toxin alone does not cause intestinal necrosis, indicating that other virulence factors might be needed to cause the extensive tissue necrosis observed in necro-haemorrhagic enteritis. This review summarizes recent research that has increased our understanding of the pathogenesis of bovine necro-haemorrhagic enteritis and provides information that is indispensable for the development of novel control strategies, including vaccines.
Figures
Similar articles
-
Toxin-neutralizing antibodies protect against Clostridium perfringens-induced necrosis in an intestinal loop model for bovine necrohemorrhagic enteritis.BMC Vet Res. 2016 Jun 13;12(1):101. doi: 10.1186/s12917-016-0730-8. BMC Vet Res. 2016. PMID: 27297520 Free PMC article. Clinical Trial.
-
Non-toxic perfringolysin O and α-toxin derivatives as potential vaccine candidates against bovine necrohaemorrhagic enteritis.Vet J. 2016 Nov;217:89-94. doi: 10.1016/j.tvjl.2016.09.008. Epub 2016 Oct 3. Vet J. 2016. PMID: 27810219
-
The synergistic necrohemorrhagic action of Clostridium perfringens perfringolysin and alpha toxin in the bovine intestine and against bovine endothelial cells.Vet Res. 2013 Jun 19;44(1):45. doi: 10.1186/1297-9716-44-45. Vet Res. 2013. PMID: 23782465 Free PMC article.
-
Necrotic enteritis in broilers: an updated review on the pathogenesis.Avian Pathol. 2011 Aug;40(4):341-7. doi: 10.1080/03079457.2011.590967. Avian Pathol. 2011. PMID: 21812711 Review.
-
The pathogenesis of necrotic enteritis in chickens: what we know and what we need to know: a review.Avian Pathol. 2016 Jun;45(3):288-94. doi: 10.1080/03079457.2016.1139688. Avian Pathol. 2016. PMID: 26813023 Review.
Cited by
-
The cultivable autochthonous microbiota of the critically endangered Northern bald ibis (Geronticus eremita).PLoS One. 2018 Apr 4;13(4):e0195255. doi: 10.1371/journal.pone.0195255. eCollection 2018. PLoS One. 2018. PMID: 29617453 Free PMC article.
-
Potential Determinants of Clostridium Spp. Occurrence in Polish Silage.J Vet Res. 2020 Nov 6;64(4):549-555. doi: 10.2478/jvetres-2020-0075. eCollection 2020 Dec. J Vet Res. 2020. PMID: 33367144 Free PMC article.
-
Necrotic Enteritis in Broiler Chickens: A Review on the Pathogen, Pathogenesis, and Prevention.Microorganisms. 2022 Sep 30;10(10):1958. doi: 10.3390/microorganisms10101958. Microorganisms. 2022. PMID: 36296234 Free PMC article. Review.
-
A Comprehensive Review of the Common Bacterial Infections in Dairy Calves and Advanced Strategies for Health Management.Vet Med (Auckl). 2024 Jan 24;15:1-14. doi: 10.2147/VMRR.S452925. eCollection 2024. Vet Med (Auckl). 2024. PMID: 38288284 Free PMC article. Review.
-
Role of Clostridium perfringens Necrotic Enteritis B-like Toxin in Disease Pathogenesis.Vaccines (Basel). 2021 Dec 31;10(1):61. doi: 10.3390/vaccines10010061. Vaccines (Basel). 2021. PMID: 35062722 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

