Human DPSCs fabricate vascularized woven bone tissue: a new tool in bone tissue engineering
- PMID: 28209631
- PMCID: PMC5383003
- DOI: 10.1042/CS20170047
Human DPSCs fabricate vascularized woven bone tissue: a new tool in bone tissue engineering
Abstract
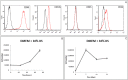
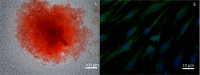
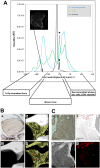
Human dental pulp stem cells (hDPSCs) are mesenchymal stem cells that have been successfully used in human bone tissue engineering. To establish whether these cells can lead to a bone tissue ready to be grafted, we checked DPSCs for their osteogenic and angiogenic differentiation capabilities with the specific aim of obtaining a new tool for bone transplantation. Therefore, hDPSCs were specifically selected from the stromal-vascular dental pulp fraction, using appropriate markers, and cultured. Growth curves, expression of bone-related markers, calcification and angiogenesis as well as an in vivo transplantation assay were performed. We found that hDPSCs proliferate, differentiate into osteoblasts and express high levels of angiogenic genes, such as vascular endothelial growth factor and platelet-derived growth factor A. Human DPSCs, after 40 days of culture, give rise to a 3D structure resembling a woven fibrous bone. These woven bone (WB) samples were analysed using classic histology and synchrotron-based, X-ray phase-contrast microtomography and holotomography. WB showed histological and attractive physical qualities of bone with few areas of mineralization and neovessels. Such WB, when transplanted into rats, was remodelled into vascularized bone tissue. Taken together, our data lead to the assumption that WB samples, fabricated by DPSCs, constitute a noteworthy tool and do not need the use of scaffolds, and therefore they are ready for customized regeneration.
Keywords: bone differentiation; bone regeneration; bone tissue engineering; hDPSCs; holotomography; human Dental Pulp Stem Cells; human serum; phc-microCT; woven bon; woven bone.
© 2017 The Author(s); published by Portland Press Limited on behalf of the Biochemical Society.
Figures





References
-
- Ferro F., Spelat R., Beltrami A.P., Cesselli D. and Curcio F. (2012) Isolation and characterization of human dental pulp derived stem cells by using media containing low human serum percentage as clinical grade substitutes for bovine serum. PLoS One 7, e48945.10.1371/journal.pone.0048945 - DOI - PMC - PubMed
-
- d'Aquino R., Graziano A., Sampaolesi M., Laino G., Pirozzi G., De Rosa A.. et al. (2007) Human postnatal dental pulp cells codifferentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 14, 1162–117110.1038/sj.cdd.4402121 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

