Doublet stimulation increases Ca2+ binding to troponin C to ensure rapid force development in skeletal muscle
- PMID: 28209802
- PMCID: PMC5339514
- DOI: 10.1085/jgp.201611727
Doublet stimulation increases Ca2+ binding to troponin C to ensure rapid force development in skeletal muscle
Abstract
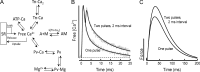
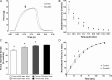
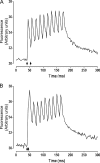
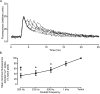
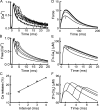
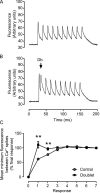
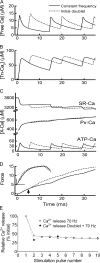
Fast-twitch skeletal muscle fibers are often exposed to motor neuron double discharges (≥200 Hz), which markedly increase both the rate of contraction and the magnitude of the resulting force responses. However, the mechanism responsible for these effects is poorly understood, likely because of technical limitations in previous studies. In this study, we measured cytosolic Ca2+ during doublet activation using the low-affinity indicator Mag-Fluo-4 at high temporal resolution and modeled the effects of doublet stimulation on sarcoplasmic reticulum (SR) Ca2+ release, binding of Ca2+ to cytosolic buffers, and force enhancement in fast-twitch fibers. Single isolated fibers respond to doublet pulses with two clear Ca2+ spikes, at doublet frequencies up to 1 KHz. A 200-Hz doublet at the start of a tetanic stimulation train (70 Hz) decreases the drop in free Ca2+ between the first three Ca2+ spikes of the transient, maintaining a higher overall free Ca2+ level during first 20-30 ms of the response. Doublet stimulation also increased the rate of force development in isolated fast-twitch muscles. We also modeled SR Ca2+ release rates during doublet stimulation and showed that Ca2+-dependent inactivation of ryanodine receptor activity is rapid, occurring ≤1ms after initial release. Furthermore, we modeled Ca2+ binding to the main intracellular Ca2+ buffers of troponin C (TnC), parvalbumin, and the SR Ca2+ pump during Ca2+ release and found that the main effect of the second response in the doublet is to more rapidly increase the occupation of the second Ca2+-binding site on TnC (TnC2), resulting in earlier activation of force. We conclude that doublet stimulation maintains high cytosolic Ca2+ levels for longer in the early phase of the Ca2+ response, resulting in faster saturation of TnC2 with Ca2+, faster initiation of cross-bridge cycling, and more rapid force development.
© 2017 Bakker et al.
Figures
Comment in
-
Acting on an impulse (or two): Advantages of high-frequency tetanic onset in skeletal muscle.J Gen Physiol. 2017 Mar 6;149(3):297-300. doi: 10.1085/jgp.201711763. Epub 2017 Feb 17. J Gen Physiol. 2017. PMID: 28213459 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

