Salicylic Acid-Dependent Plant Stress Signaling via Mitochondrial Succinate Dehydrogenase
- PMID: 28209841
- PMCID: PMC5373042
- DOI: 10.1104/pp.16.00060
Salicylic Acid-Dependent Plant Stress Signaling via Mitochondrial Succinate Dehydrogenase
Abstract
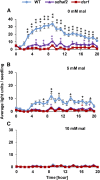
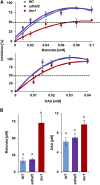
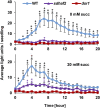
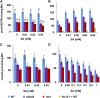
Mitochondria are known for their role in ATP production and generation of reactive oxygen species, but little is known about the mechanism of their early involvement in plant stress signaling. The role of mitochondrial succinate dehydrogenase (SDH) in salicylic acid (SA) signaling was analyzed using two mutants: disrupted in stress response1 (dsr1), which is a point mutation in SDH1 identified in a loss of SA signaling screen, and a knockdown mutant (sdhaf2) for SDH assembly factor 2 that is required for FAD insertion into SDH1. Both mutants showed strongly decreased SA-inducible stress promoter responses and low SDH maximum capacity compared to wild type, while dsr1 also showed low succinate affinity, low catalytic efficiency, and increased resistance to SDH competitive inhibitors. The SA-induced promoter responses could be partially rescued in sdhaf2, but not in dsr1, by supplementing the plant growth media with succinate. Kinetic characterization showed that low concentrations of either SA or ubiquinone binding site inhibitors increased SDH activity and induced mitochondrial H2O2 production. Both dsr1 and sdhaf2 showed lower rates of SA-dependent H2O2 production in vitro in line with their low SA-dependent stress signaling responses in vivo. This provides quantitative and kinetic evidence that SA acts at or near the ubiquinone binding site of SDH to stimulate activity and contributes to plant stress signaling by increased rates of mitochondrial H2O2 production, leading to part of the SA-dependent transcriptional response in plant cells.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures

References
-
- Andreyev AY, Kushnareva YE, Starkov AA (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70: 200–214 - PubMed
-
- Bi YM, Kenton P, Mur L, Darby R, Draper J (1995) Hydrogen peroxide does not function downstream of salicylic acid in the induction of PR protein expression. Plant J 8: 235–245 - PubMed
-
- Bienert GP, Chaumont F (2014) Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys Acta 1840: 1596–1604 - PubMed
-
- Bienert GP, Møller AL, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282: 1183–1192 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

