GTPase activity-coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis
- PMID: 28209899
- PMCID: PMC5851775
- DOI: 10.1126/science.aak9995
GTPase activity-coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis
Abstract
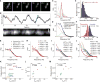
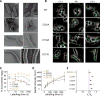
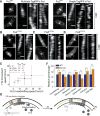
The bacterial tubulin FtsZ is the central component of the cell division machinery, coordinating an ensemble of proteins involved in septal cell wall synthesis to ensure successful constriction. How cells achieve this coordination is unknown. We found that in Escherichia coli cells, FtsZ exhibits dynamic treadmilling predominantly determined by its guanosine triphosphatase activity. The treadmilling dynamics direct the processive movement of the septal cell wall synthesis machinery but do not limit the rate of septal synthesis. In FtsZ mutants with severely reduced treadmilling, the spatial distribution of septal synthesis and the molecular composition and ultrastructure of the septal cell wall were substantially altered. Thus, FtsZ treadmilling provides a mechanism for achieving uniform septal cell wall synthesis to enable correct polar morphology.
Copyright © 2017, American Association for the Advancement of Science.
Figures

Comment in
-
Bacterial physiology: Treadmilling runs bacterial division.Nat Rev Microbiol. 2017 Mar 13;15(4):193. doi: 10.1038/nrmicro.2017.24. Nat Rev Microbiol. 2017. PMID: 28286344 No abstract available.
-
Bacterial Division: FtsZ Treadmills to Build a Beautiful Wall.Curr Biol. 2017 Apr 24;27(8):R301-R303. doi: 10.1016/j.cub.2017.03.019. Curr Biol. 2017. PMID: 28441562
References
-
- Nogales E, Downing KH, Amos LA, Löwe J. Nat Struct Biol. 1998;5:451–458. - PubMed
-
- Vaughan S, Wickstead B, Gull K, Addinall SG. J Mol Evol. 2004;58:19–29. - PubMed
-
- Bi EF, Lutkenhaus J. Nature. 1991;354:161–164. - PubMed
-
- Egan AJ, Vollmer W. Ann NY Acad Sci. 2013;1277:8–28. - PubMed
-
- de Boer P, Crossley R, Rothfield L. Nature. 1992;359:254–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

