Exclusive destruction of mitotic spindles in human cancer cells
- PMID: 28209915
- PMCID: PMC5400547
- DOI: 10.18632/oncotarget.15343
Exclusive destruction of mitotic spindles in human cancer cells
Erratum in
-
Correction: Exclusive destruction of mitotic spindles in human cancer cells.Oncotarget. 2020 Apr 7;11(14):1290-1291. doi: 10.18632/oncotarget.27499. eCollection 2020 Apr 7. Oncotarget. 2020. PMID: 32292578 Free PMC article.
Abstract
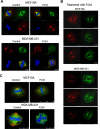
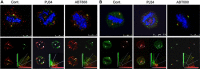
We identified target proteins modified by phenanthrenes that cause exclusive eradication of human cancer cells. The cytotoxic activity of the phenanthrenes in a variety of human cancer cells is attributed by these findings to post translational modifications of NuMA and kinesins HSET/kifC1 and kif18A. Their activity prevented the binding of NuMA to α-tubulin and kinesins in human cancer cells, and caused aberrant spindles. The most efficient cytotoxic activity of the phenanthridine PJ34, caused significantly smaller aberrant spindles with disrupted spindle poles and scattered extra-centrosomes and chromosomes. Concomitantly, PJ34 induced tumor growth arrest of human malignant tumors developed in athymic nude mice, indicating the relevance of its activity for cancer therapy.
Keywords: NuMA; human cancer cells; kinesins; mitotic spindles; phenanthrenes.
Conflict of interest statement
The authors declare no competing financial interests. M.C-A. is the inventor in patents US 8,729,080 B2 and EP 2 207 548B1.
Figures






References
-
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. - PubMed
-
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. - PubMed
-
- Mangerich A, Bürkle A. How to kill tumor cells with inhibitors of poly(ADP-ribosyl)- ation. Int J Cancer. 2011;128:251–265. - PubMed
-
- Jagtap P, Szabo C. Poly(ADP-ribose)polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discovery. 2005;4:421–440. - PubMed
-
- Passeri D, Camaioni E, Liscio P, Sabbatini P, Ferri M, Carotti A, Giacchè N, Pellicciari R, Gioiello A, Macchiarulo A. Concepts and Molecular Aspects in the Polypharmacology of PARP-1 Inhibitors. ChemMedChem. 2016;11:1219–1226. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

