A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything
- PMID: 28210243
- PMCID: PMC5288344
- DOI: 10.3389/fmicb.2017.00108
A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything
Abstract
Real time PCR (quantitative PCR, qPCR) is now a well-established method for the detection, quantification, and typing of different microbial agents in the areas of clinical and veterinary diagnostics and food safety. Although the concept of PCR is relatively simple, there are specific issues in qPCR that developers and users of this technology must bear in mind. These include the use of correct terminology and definitions, understanding of the principle of PCR, difficulties with interpretation and presentation of data, the limitations of qPCR in different areas of microbial diagnostics and parameters important for the description of qPCR performance. It is not our intention in this review to describe every single aspect of qPCR design, optimization, and validation; however, it is our hope that this basic guide will help to orient beginners and users of qPCR in the use of this powerful technique.
Keywords: accuracy; limit of detection; limit of quantification; precision; quantitative PCR; trueness.
Figures
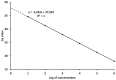
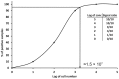
References
-
- Anonymous (1994). ISO 5725-1 - Accuracy (Trueness and Precision) of Measurement Methods and Results — Part 1: General Principles and Definitions. Geneva: ISO - International Organization for Standardization.
-
- Anonymous (2009). Protocol for the Validation of Alternative Microbiological Methods. Søborg: NordVal International /Nordic Committee on Food Analysis, NMKL.
-
- Anonymous (2011). ISO 16140 - Microbiology of Food and Animal Feeding Stuffs - Protocol for the Validation of Alternative Methods. Geneva: ISO - International Organization for Standardization.
-
- Anonymous (2012). Methods Committee Guidelines for Validation of Microbiological Methods for Food and Environmental Surfaces. Rockville, MD: AOAC INTERNATIONAL.
-
- Anonymous R. (2013). Terrestrial Manual, 7th Edn, Chapter 1.1.5. Principles and Methods of Validation of Diagnostic Assays for Infectious Diseases (Version adopted in May, 2013). Paris: World Organisation for Animal Health.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

