Time course of glatiramer acetate efficacy in patients with RRMS in the GALA study
- PMID: 28210662
- PMCID: PMC5299631
- DOI: 10.1212/NXI.0000000000000327
Time course of glatiramer acetate efficacy in patients with RRMS in the GALA study
Abstract
Objective: To determine the time to efficacy onset of glatiramer acetate (GA) 40 mg/mL 3-times-weekly formulation (GA40).
Methods: This post hoc analysis of data from the 1-year, double-blind, placebo-controlled phase of the Glatiramer Acetate Low-Frequency Administration study (NCT01067521) of GA40 in patients with relapsing-remitting MS (RRMS) sought to determine the timing of efficacy onset using a novel data-censoring approach.
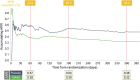
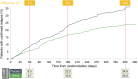
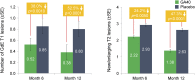
Results: Compared with placebo-treated patients, those receiving GA40 exhibited a >30% reduction in the accumulated annualized relapse rate (ARR) within 2 months of initiating treatment and generally sustained this treatment difference during the 1-year study. Similarly, the proportion of GA40-treated patients who remained relapse-free was distinctly greater by month 2 and continued to increase up to a 10.8% difference at the end of the study. In addition, GA40 treatment was associated with a significant reduction in the number of gadolinium-enhancing T1 lesions and new/enlarging T2 lesions by month 6, with full treatment effect observed after 1 year.
Conclusions: GA40 contributes to efficacy within 2 months of the start of treatment in patients with RRMS. These results are consistent with the observed time to efficacy onset for patients treated with GA 20 mg/mL daily in previous randomized, placebo-controlled clinical trials.
Classification of evidence: This study provides Class II evidence that for patients with RRMS, a 3-times-weekly formulation of GA 40 mg/mL leads to a >30% reduction in the ARR within 2 months.
Figures

References
-
- Boster AL, Ford CC, Neudorfer O, Gilgun-Sherki Y. Glatiramer acetate: long-term safety and efficacy in relapsing-remitting multiple sclerosis. Expert Rev Neurother 2015;15:575–586. - PubMed
-
- Copaxone [Prescribing Information]. Overland Park, KS: TEVA Neuroscience, Inc.; 2014.
-
- Arnal-Garcia C, Amigo-Jorrin Mdel C, Lopez-Real AM, et al. . Long-term effectiveness of glatiramer acetate in clinical practice conditions. J Clin Neurosci 2014;21:2212–2218. - PubMed
-
- Debouverie M, Moreau T, Lebrun C, Heinzlef O, Brudon F, Msihid J. A longitudinal observational study of a cohort of patients with relapsing-remitting multiple sclerosis treated with glatiramer acetate. Eur J Neurol 2007;14:1266–1274. - PubMed
-
- Rovaris M, Codella M, Moiola L, et al. . Effect of glatiramer acetate on MS lesions enhancing at different gadolinium doses. Neurology 2002;59:1429–1432. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
