IL33 Is a Stomach Alarmin That Initiates a Skewed Th2 Response to Injury and Infection
- PMID: 28210674
- PMCID: PMC5301136
- DOI: 10.1016/j.jcmgh.2014.12.003
IL33 Is a Stomach Alarmin That Initiates a Skewed Th2 Response to Injury and Infection
Abstract
Background & aims: Interleukin (IL)33 is a recently described alarmin that is highly expressed in the gastric mucosa and potently activates Th2 immunity. It may play a pivotal role during Helicobacter pylori infection. Here, we delineate the role of IL33 in the normal gastric mucosa and in response to gastropathy.
Methods: IL33 expression was evaluated in mice and human biopsy specimens infected with H pylori and in mice after dosing with aspirin. IL33 expression was localized in the gastric mucosa using immunofluorescence. Mice were given 1 or 7 daily doses of recombinant IL33 (1 μg/dose), and the stomach and the spleen responses were quantified morphologically, by flow cytometry and using quantitative reverse-transcription polymerase chain reaction and immunoblotting.
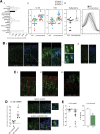
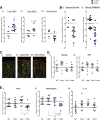
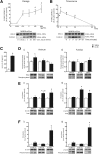
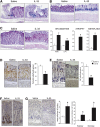
Results: In mice, the IL33 protein was localized to the nucleus of a subpopulation of surface mucus cells, and co-localized with the surface mucus cell markers Ulex Europaeus 1 (UEA1), and Mucin 5AC (Muc5AC). A small proportion of IL33-positive epithelial cells also were Ki-67 positive. IL33 and its receptor Interleukin 1 receptor-like 1 (ST2) were increased 4-fold after acute (1-day) H pylori infection, however, this increase was not apparent after 7 days and IL33 expression was reduced 2-fold after 2 months. Similarly, human biopsy specimens positive for H pylori had a reduced IL33 expression. Chronic IL33 treatment in mice caused systemic activation of innate lymphoid cell 2 and polarization of macrophages to the M2 phenotype. In the stomach, IL33-treated mice developed transmural inflammation and mucous metaplasia that was mediated by Th2/signal transducer and activator of transcription 3 signaling. Rag-1-/- mice, lacking mature lymphocytes, were protected from IL33-induced gastric pathology.
Conclusions: IL33 is highly expressed in the gastric mucosa and promotes the activation of T helper 2-cytokine-expressing cells. The loss of IL33 expression after prolonged H pylori infection may be permissive for the T helper 1-biased immune response observed during H pylori infection and subsequent precancerous progression.
Keywords: AB, Alcian blue; DC, dendritic cell; ELISA, enzyme-linked immunosorbent assay; ERK, extracellular signal–regulated kinase; FBS, fetal bovine serum; Gastric Cancer; HBSS, Hank’s balanced salt solution; Helicobacter pylori; IL, interleukin; IL33; ILC, innate lymphoid cell; Inflammatory Response; NF-κB, nuclear factor-κB; PAS, periodic acid–Schiff; PCR, polymerase chain reaction; QRT-PCR, quantitative reverse-transcription polymerase chain reaction; SMC, surface mucus cells; SPF, specific pathogen free; SS1, Sydney strain 1; STAT, signal transducer and activator of transcription; TFF, trefoil factor; Th, T-helper; WT, wild type; mRNA, messenger RNA.
Figures


References
-
- Oppenheim J.J., Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. - PubMed
-
- Arshad M.I., Piquet-Pellorce C., Samson M. IL-33 and HMGB1 alarmins: sensors of cellular death and their involvement in liver pathology. Liver Int. 2012;32:1200–1210. - PubMed
-
- Schmitz J., Owyang A., Oldham E. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

