Modulation of Hepatic Protein Kinase Cβ Expression in Metabolic Adaptation to a Lithogenic Diet
- PMID: 28210689
- PMCID: PMC5301293
- DOI: 10.1016/j.jcmgh.2015.05.008
Modulation of Hepatic Protein Kinase Cβ Expression in Metabolic Adaptation to a Lithogenic Diet
Abstract
Background & aims: Dietary factors are likely an important determinant of gallstone development, and difficulty in adapting to lithogenic diets may predispose individuals to gallstone formation. Identification of the critical early diet-dependent metabolic markers of adaptability is urgently needed to prevent gallstone development. We focus on the interaction between diet and genes, and the resulting potential to influence gallstone risk by dietary modification.
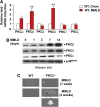
Methods: Expression levels of hepatic protein kinase C (PKC) isoforms were determined in lithogenic diet-fed mice, and the relationship of hepatic cholesterol content and PKCβ expression and the effect of hepatic PKCβ overexpression on intracellular signaling pathways were analyzed.
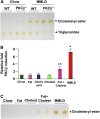
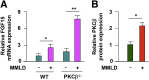
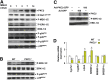
Results: Lithogenic diet feeding resulted in a striking induction of hepatic PKCβ and PKCδ mRNA and protein levels, which preceded the appearance of biliary cholesterol crystals. Unlike PKCβ deficiency, global PKCδ deficiency did not influence lithogenic diet-induced gallstone formation. Interestingly, a deficiency of apolipoprotein E abrogated the diet-induced hepatic PKCβ expression, whereas a deficiency of liver X receptor-α further potentiated the induction, suggesting a potential link between the degree of hepatic PKCβ induction and the intracellular cholesterol content. Furthermore, our results suggest that PKCβ is a physiologic repressor of ileum basal fibroblast growth factor 15 (FGF15) expression and activity of hepatic proto-oncogene serine/threonine-protein kinase Raf-1/mitogen-activated protein (MAP) kinase kinase/extracellular signal-regulated kinases 1/2 (Raf-1/MEK/ERK1/2) cascade proteins, and the complex interactions between these pathways may determine the degree of hepatic ERK1/2 activation, a potent suppressor of cholesterol 7α-hydroxylase and sterol 12α-hydroxylase expression. We found that PKCβ regulated Raf-1 activity by modulating the inhibitory Raf-1Ser259 phosphorylation.
Conclusions: Our results demonstrate a novel interaction between the hepatic PKCβ/Raf-1 regulatory axis and ileum PKCβ/FGF15/ERK axis, which could modulate the bile lithogenecity of dietary lipids. The data presented are consistent with a two-pronged mechanism by which intestine and liver PKCβ signaling converges on the liver ERK1/2 pathway to control the hepatic adaptive response to a lithogenic diet. Elucidating the impact and the underlying mechanism(s) of PKCβ action could help us understand how different types of dietary fat modify the risk of gallstone formation, information that could help to identify novel targets for therapeutic approaches to combat this disease.
Keywords: Akt, protein kinase B; ApoE, apolipoprotein E; Cyp7a1, cholesterol 7α-hydroxylase; Cyp8b1, sterol 12α-hydroxylase; ERK1/2, extracellular signal regulated kinase-1/2; FGF15, fibroblast growth factor 15; FXR, farnesoid X receptor; GSK-3, glycogen synthase kinase-3; Hepatic Cholesterol Metabolism; JNK, c-Jun N-terminal kinase; LDL, low-density lipoprotein; LXR, liver X receptor; Lithogenic Diet; MEK, mitogen-activated protein (MAP) kinase kinase; MMLD, modified milk fat lithogenic diet; PKCβ, protein kinase C isoform β; Protein Kinase Cβ; Raf-1, Raf-1 hepatic proto-oncogene serine/threonine-protein kinase; SREBP, sterol response element-binding protein; Signal Transduction; WT, wild type.
Figures


Similar articles
-
PKCβ: Expanding role in hepatic adaptation of cholesterol homeostasis to dietary fat/cholesterol.Am J Physiol Gastrointest Liver Physiol. 2017 Mar 1;312(3):G266-G273. doi: 10.1152/ajpgi.00373.2016. Epub 2017 Jan 19. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28104587 Free PMC article. Review.
-
Disruption of the murine protein kinase Cbeta gene promotes gallstone formation and alters biliary lipid and hepatic cholesterol metabolism.J Biol Chem. 2011 Jul 1;286(26):22795-805. doi: 10.1074/jbc.M111.250282. Epub 2011 May 5. J Biol Chem. 2011. PMID: 21550971 Free PMC article.
-
Proteasome inhibition protects against diet-induced gallstone formation through modulation of cholesterol and bile acid homeostasis.Int J Mol Med. 2018 Mar;41(3):1715-1723. doi: 10.3892/ijmm.2017.3326. Epub 2017 Dec 15. Int J Mol Med. 2018. PMID: 29286073
-
Deficiency of Niemann-Pick C1 protein protects against diet-induced gallstone formation in mice.Liver Int. 2010 Jul;30(6):887-97. doi: 10.1111/j.1478-3231.2010.02230.x. Epub 2010 Apr 8. Liver Int. 2010. PMID: 20408952
-
Bile acids and sphingosine-1-phosphate receptor 2 in hepatic lipid metabolism.Acta Pharm Sin B. 2015 Mar;5(2):151-7. doi: 10.1016/j.apsb.2014.12.009. Epub 2015 Feb 9. Acta Pharm Sin B. 2015. PMID: 26579441 Free PMC article. Review.
Cited by
-
PKCβ: Expanding role in hepatic adaptation of cholesterol homeostasis to dietary fat/cholesterol.Am J Physiol Gastrointest Liver Physiol. 2017 Mar 1;312(3):G266-G273. doi: 10.1152/ajpgi.00373.2016. Epub 2017 Jan 19. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28104587 Free PMC article. Review.
-
Dietary fat/cholesterol-sensitive PKCβ-RB signaling: Potential role in NASH/HCC axis.Oncotarget. 2017 May 15;8(43):73757-73765. doi: 10.18632/oncotarget.17890. eCollection 2017 Sep 26. Oncotarget. 2017. PMID: 29088742 Free PMC article.
-
Protein Kinase Cβ: Linking Intestine Fibroblast Growth Factor 15 to Hepatic Extracellular Signal Regulated Kinase 1/2 Signaling in Bile Acid and Cholesterol Homeostasis.Cell Mol Gastroenterol Hepatol. 2015 Jun 5;1(4):350-351. doi: 10.1016/j.jcmgh.2015.05.003. eCollection 2015 Jul. Cell Mol Gastroenterol Hepatol. 2015. PMID: 28210685 Free PMC article. No abstract available.
-
Role of hepatic PKCβ in nutritional regulation of hepatic glycogen synthesis.JCI Insight. 2021 Oct 8;6(19):e149023. doi: 10.1172/jci.insight.149023. JCI Insight. 2021. PMID: 34622807 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

