Granulocyte Macrophage Colony-Stimulating Factor-Activated CD39+/CD73+ Murine Monocytes Modulate Intestinal Inflammation via Induction of Regulatory T Cells
- PMID: 28210690
- PMCID: PMC5301274
- DOI: 10.1016/j.jcmgh.2015.04.005
Granulocyte Macrophage Colony-Stimulating Factor-Activated CD39+/CD73+ Murine Monocytes Modulate Intestinal Inflammation via Induction of Regulatory T Cells
Abstract
Background & aims: Granulocyte macrophage colony-stimulating factor (GM-CSF) treatment induces clinical response in patients with active Crohn's disease. To explore whether monocytes mediate GM-CSF effects in vivo, we used a mouse model of chronic colitis induced by dextran sulfate sodium (DSS).
Methods: Murine bone marrow-derived monocytes were activated with GM-CSF in vitro, and gene expression, phenotype, and function of GM-CSF-activated monocytes (GMaM) were analyzed. Therapeutic effects of GMaM were assessed in a model of chronic colitis induced by repeated cycles of DSS. Monocytes were administered intravenously and their immunomodulatory functions were evaluated in vivo by clinical monitoring, histology, endoscopy, immunohistochemistry, and expression of inflammatory markers in the colon. The distribution of injected monocytes in the intestine was measured by in vivo imaging.
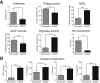
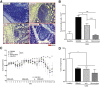
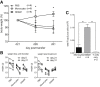
Results: GMaM expressed significantly higher levels of anti-inflammatory molecules. Production of reactive oxygen species was also increased while phagocytosis and adherence were decreased. GMaM up-regulated CD39 and CD73, which allows the conversion of adenosine triphosphate into adenosine and coincided with the induction of Foxp3+ (forkhead-box-protein P3 positive) regulatory T cells (Treg) in cocultures of GMaM and naive T cells. In chronic DSS-induced colitis, adoptive transfer of GMaM led to significant clinical improvement, as demonstrated by reduced weight loss, inflammatory infiltration, ulceration, and colon shrinkage. As GMaM migrated faster and persisted longer in the inflamed intestine compared with control monocytes, their presence induced Treg generation in vivo.
Conclusions: GM-CSF leads to specific monocyte activation that modulates experimental colitis via mechanisms that include the induction of Treg. We demonstrate a possible mechanism of Treg induction through CD39 and CD73 expression on monocytes.
Keywords: ALDH, aldehyde dehydrogenase; ATP, adenosine triphosphate; Adaptive Immunity; Arg1, arginase 1; CD, Crohn’s disease; CD39, E-NTPDase; CD73, ecto-5′-nucleotidase; CFSE, carboxyfluorescein succinimidyl ester; DC, dendritic cells; DSS, dextran sulfate sodium; Dextran Sulfate Sodium; Experimental Colitis; FCS, fetal calf serum; Foxp3, forkhead-box-protein P3; GM-CSF; GM-CSF, granulocyte macrophage colony-stimulating factor; GMaM, granulocyte-macrophage colony-stimulating factor–activated monocytes; IBD, inflammatory bowel disease; IL, interleukin; IL-1Ra, IL-1 receptor antagonist; Immune Response; Innate Immunity; LPS, lipopolysaccharide; MACS, magnetic-activated cell sorting; MEICS, murine endoscopic index of colitis severity; Monocyte; NO, nitric oxide; OD, optical density; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; RA, retinoic acid; ROS, reactive oxygen species; T Cell; TNFα, tumor necrosis factor α; Treg, regulatory T cells; WT, wild type; qRT-PCR, quantitative reverse-transcription polymerase chain reaction.
Figures





References
-
- Di Sabatino A., Biancheri P., Rovedatti L. New pathogenic paradigms in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:368–371. - PubMed
-
- Dabritz J. Granulocyte macrophage colony-stimulating factor and the intestinal innate immune cell homeostasis in Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2014;306:G455–G465. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

