Toll-like receptor signaling and stages of addiction
- PMID: 28210782
- PMCID: PMC5420377
- DOI: 10.1007/s00213-017-4560-6
Toll-like receptor signaling and stages of addiction
Abstract
Background: Athina Markou and her colleagues discovered persistent changes in adult behavior following adolescent exposure to ethanol or nicotine consistent with increased risk for developing addiction. Building on Dr. Markou's important work and that of others in the field, researchers at the Bowles Center for Alcohol Studies have found that persistent changes in behavior following adolescent stress or alcohol exposure may be linked to induction of immune signaling in brain.
Aim: This study aims to illuminate the critical interrelationship of the innate immune system (e.g., toll-like receptors [TLRs], high-mobility group box 1 [HMGB1]) in the neurobiology of addiction.
Method: This study reviews the relevant research regarding the relationship between the innate immune system and addiction.
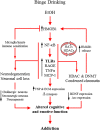
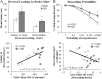
Conclusion: Emerging evidence indicates that TLRs in brain, particularly those on microglia, respond to endogenous innate immune agonists such as HMGB1 and microRNAs (miRNAs). Multiple TLRs, HMGB1, and miRNAs are induced in the brain by stress, alcohol, and other drugs of abuse and are increased in the postmortem human alcoholic brain. Enhanced TLR-innate immune signaling in brain leads to epigenetic modifications, alterations in synaptic plasticity, and loss of neuronal cell populations, which contribute to cognitive and emotive dysfunctions. Addiction involves progressive stages of drug binges and intoxication, withdrawal-negative affect, and ultimately compulsive drug use and abuse. Toll-like receptor signaling within cortical-limbic circuits is modified by alcohol and stress in a manner consistent with promoting progression through the stages of addiction.
Keywords: Alcohol use disorder; Cytokines; HMGB1; miRNA let-7.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

