Updated results from GEST study: a randomized, three-arm phase III study for advanced pancreatic cancer
- PMID: 28210843
- PMCID: PMC5427167
- DOI: 10.1007/s00432-017-2349-y
Updated results from GEST study: a randomized, three-arm phase III study for advanced pancreatic cancer
Abstract
Purpose: The GEST study showed non-inferiority of S-1 but not superiority of gemcitabine plus S-1 (GS) to gemcitabine alone for overall survival with the data by the cut-off date of 31st July in 2010 for chemo-naïve patients with advanced pancreatic cancer. We considered it important to determine whether S-1 maintains non-inferiority after a long-term follow-up in the GEST study and to obtain a firm positive conclusion. In addition, it may be an interesting challenge to explore the efficacious profile of GS in the long-term follow-up study. Using the data from the follow-up period, background and efficacy in patients from Taiwan and Japan, as well as the rates of tumor shrinkage in locally advanced and metastatic patients (Waterfall plot) were also analyzed.
Methods: The results of the primary analysis were reconfirmed, and subset analysis of overall survival and progression-free survival was performed based on the overall survival data updated by the cut-off date of 31st July in 2011.
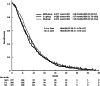
Results: The median follow-up period was 29.8 months, and 795 deaths occurred (95.6%). The median overall survival was 8.8 months for gemcitabine, 9.7 months for S-1 (hazard ratio [HR], 0.96; 97.5% confidence interval [CI], 0.79-1.17), and 9.9 months for GS (HR 0.91; 97.5% CI 0.75-1.11). In patients with performance status (PS) 0, the median overall survival was 9.8 months for gemcitabine, 10.9 months for S-1, and 10.5 months for GS. In patients with PS 1, the median overall survival was 6.2 months for gemcitabine, 6.3 months for S-1, and 9.6 months for GS.
Conclusion: Our survey reconfirmed the non-inferiority of S-1 to gemcitabine and showed S-1 can be used as one of the standard treatment options for advanced pancreatic cancer.
Trial registration: ClinicalTrials.gov: NCT00498225.
Keywords: Gemcitabine; Pancreatic cancer; S-1; Subgroup analysis; Updated data.
Conflict of interest statement
TO has received consulting fees, honoraria, and research funding from Taiho Pharmaceutical Co. Ltd. (Taiho) and Eli Lilly and Company (Eli Lilly). SM has received honoraria from Taiho and Eli Lilly. HU has received consulting fees and research funding from Taiho; honoraria from Taiho and Eli Lilly. TI has received consulting fees, honoraria, and research funding from Taiho. NB has received honoraria and research funding from Taiho. TH has received consulting fees and research funding from Taiho. JF has received consulting fees from Taiho; honoraria and research funding from Taiho and Eli Lilly. KM has received consulting fees and research funding from Taiho. SO has received consulting fees, honoraria and research funding from Taiho. TY has received consulting fees and research funding from Taiho. KY has received consulting fees and research funding from Taiho; honoraria from Taiho and Eli Lilly. AF has received consulting fees, honoraria, and research funding from Taiho. JSC has received consulting fees from TTY Biopharm Co. Ltd. (TTY), Eli Lilly, Novartis, and Bayer. ALC has received consultant fees from Boehringer Ingelheim, Sanofi K.K., and TTY; honoraria from Novartis, Bayer and Merck Sharp & Dohme(MSD); research funding from Sanofi K.K. AS has received consulting fees and honoraria from Taiho. YO has received consulting fees from Taiho; honoraria from Eli Lilly. All remaining authors have declared no conflicts of interest.
Figures


References
-
- Brookmeyer R, Crowley J (1982) A confidence interval for the median survival time. Biometrics 38:29–41
-
- Burris HA 3rd, Moore MJ, ersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413 - PubMed
-
- Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825 - PubMed
-
- Kalbfleisch JD, Prentice RL (1980) The statistical analysis of failure time data. Wiley, New York, p 14
-
- Moore MJ, Goldstein D, Hamm J et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960–1966 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

