Physiology and role of irisin in glucose homeostasis
- PMID: 28211512
- PMCID: PMC5878942
- DOI: 10.1038/nrendo.2016.221
Physiology and role of irisin in glucose homeostasis
Abstract
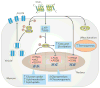
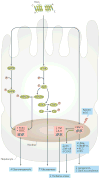
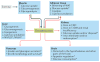
Irisin is a myokine that leads to increased energy expenditure by stimulating the 'browning' of white adipose tissue. In the first description of this hormone, increased levels of circulating irisin, which is cleaved from its precursor fibronectin type III domain-containing protein 5, were associated with improved glucose homeostasis by reducing insulin resistance. Consequently, several studies attempted to characterize the role of irisin in glucose regulation, but contradictory results have been reported, and even the existence of this hormone has been questioned. In this Review, we present the current knowledge on the physiology of irisin and its role in glucose homeostasis. We describe the mechanisms involved in the synthesis, secretion, circulation and regulation of irisin, and the controversies regarding the measurement of irisin. We also discuss the direct effects of irisin on glucose regulatory mechanisms in different organs, the indirect effects and interactions with other hormones, and the important open questions with regard to irisin in those organs. Finally, we present the results from animal interventional studies and from human clinical studies investigating the association of irisin with obesity, insulin resistance, type 2 diabetes mellitus and the metabolic syndrome.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Gerich JE. Physiology of glucose homeostasis. Diabetes Obes Metab. 2000;2:345–350. - PubMed
-
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. - PubMed
-
- NCBI. Fibronectin type III domain-containing protein 5 precursor [Rattus norvegicus] NCBI; 2016. https://www.ncbi.nlm.nih.gov/protein/NP_001257910.1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

