Motor control of Drosophila feeding behavior
- PMID: 28211791
- PMCID: PMC5315463
- DOI: 10.7554/eLife.19892
Motor control of Drosophila feeding behavior
Abstract
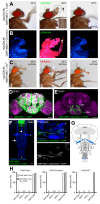
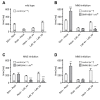
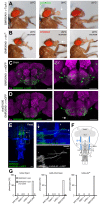
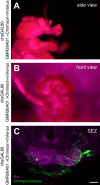
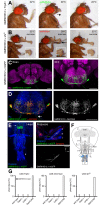
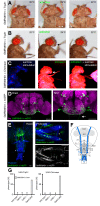
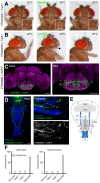
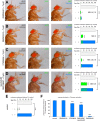
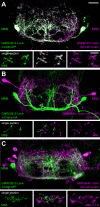
The precise coordination of body parts is essential for survival and behavior of higher organisms. While progress has been made towards the identification of central mechanisms coordinating limb movement, only limited knowledge exists regarding the generation and execution of sequential motor action patterns at the level of individual motoneurons. Here we use Drosophila proboscis extension as a model system for a reaching-like behavior. We first provide a neuroanatomical description of the motoneurons and muscles contributing to proboscis motion. Using genetic targeting in combination with artificial activation and silencing assays we identify the individual motoneurons controlling the five major sequential steps of proboscis extension and retraction. Activity-manipulations during naturally evoked proboscis extension show that orchestration of serial motoneuron activation does not rely on feed-forward mechanisms. Our data support a model in which central command circuits recruit individual motoneurons to generate task-specific proboscis extension sequences.
Keywords: D. melanogaster; feeding behavior; motoneurons; motor circuit; neuroscience.
Conflict of interest statement
KV: Senior editor,
The other authors declare that no competing interests exist.
Figures

References
-
- Altman JS, Kien J. Arthropod Brain: Its Evolution, Development Structure, and Functions. New York: Wiley; 1987. Functional organization of the subesophageal ganglion in arthropods; pp. 265–301.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

