Rictor/mTORC2 deficiency enhances keratinocyte stress tolerance via mitohormesis
- PMID: 28211872
- PMCID: PMC5384034
- DOI: 10.1038/cdd.2017.8
Rictor/mTORC2 deficiency enhances keratinocyte stress tolerance via mitohormesis
Abstract
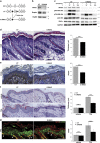
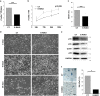
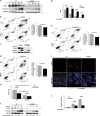
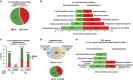
How metabolic pathways required for epidermal tissue growth and remodeling influence the ability of keratinocytes to survive stressful conditions is still largely unknown. The mechanistic target of rapamycin complex 2 (mTORC2) regulates growth and metabolism of several tissues, but its functions in epidermal cells are poorly defined. Rictor is an adaptor protein essential for mTORC2 activity. To explore the roles of mTORC2 in the epidermis, we have conditionally deleted rictor in mice via K14-Cre-mediated homologous recombination and found that its deficiency causes moderate tissue hypoplasia, reduced keratinocyte proliferation and attenuated hyperplastic response to TPA. Noteworthy, rictor-deficient keratinocytes displayed increased lifespan, protection from senescence, and enhanced tolerance to cellular stressors such as growth factors deprivation, epirubicin and X-ray in vitro and radioresistance in vivo. Rictor-deficient keratinocytes exhibited changes in global gene expression profiles consistent with metabolic alterations and enhanced stress tolerance, a shift in cell catabolic processes from glycids and lipids to glutamine consumption and increased production of mitochondrial reactive oxygen species (ROS). Mechanistically, the resiliency of rictor-deficient epidermal cells relies on these ROS increases, indicating stress resistance via mitohormesis. Thus, our findings reveal a new link between metabolic changes and stress adaptation of keratinocytes centered on mTORC2 activity, with potential implications in skin aging and therapeutic resistance of epithelial tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 2007; 6: 280–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

