A hyaluronan hydrogel scaffold-based xeno-free culture system for ex vivo expansion of human corneal epithelial stem cells
- PMID: 28211875
- PMCID: PMC5518843
- DOI: 10.1038/eye.2017.8
A hyaluronan hydrogel scaffold-based xeno-free culture system for ex vivo expansion of human corneal epithelial stem cells
Abstract
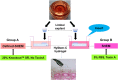
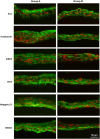
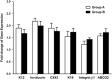
PurposeTo develop a hyaluronan hydrogel scaffold-based xeno-free culture system for ex vivo cultivation of human corneal epithelial stem cells (CESCs).Patients and MethodsCESCs were cultivated from donor limbal explants on the HyStem-C Hydrogel bio-scaffold in 12-well plates for 3 weeks. Group A used the traditional supplemented hormonal epidermal medium (SHEM) and group B used the defined SHEM (without fetal bovine serum and toxin A, adding 20% serum replacement). The growth and morphology of the cultured cells were assessed by phase contrast microscope. The expressions of specific cell markers were assessed by immunofluorescence staining and quantitative real-time PCR (qRT-PCR).ResultsSuccessful cultures of CESCs were obtained in both groups, resulting in multilayered stratified epithelia. Comparing to group A, the cells in group B was grown slightly slower and formed less cellular layers at the end of culture. The corneal specific cytokeratin (K) 12 and differentiation markers, involucrin, and connexin 43, were mainly expressed in the superficial cellular layers in both groups. Interestingly, certain basal cells were immune-positive to proposed stem cell markers such as K19, ABCG2, and integrin β1 in both groups. There was no significant difference between the two groups with regard to the gene expression levels of all these selected corneal markers (all P>0.05).ConclusionsThe hyaluronan hydrogel scaffold-based xeno-free culture system may support the expansion of regenerative CESCs without the risk of xeno component contamination. The regenerated epithelium maintains similar characteristics of native corneal epithelium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 1989; 57: 201–209. - PubMed
-
- Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci 1983; 24: 1442–1443. - PubMed
-
- Pfister RR. Corneal stem cell disease: concepts, categorization, and treatment by auto- and homotransplantation of limbal stem cells. CLAO J 1994; 20: 64–72. - PubMed
-
- Tseng SC. Concept and application of limbal stem cells. Eye 1989; 3(Pt 2): 141–157. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

