Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma
- PMID: 28212060
- PMCID: PMC5635424
- DOI: 10.1056/NEJMoa1613683
Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma
Abstract
Background: Patients with advanced urothelial carcinoma that progresses after platinum-based chemotherapy have a poor prognosis and limited treatment options.
Methods: In this open-label, international, phase 3 trial, we randomly assigned 542 patients with advanced urothelial cancer that recurred or progressed after platinum-based chemotherapy to receive pembrolizumab (a highly selective, humanized monoclonal IgG4κ isotype antibody against programmed death 1 [PD-1]) at a dose of 200 mg every 3 weeks or the investigator's choice of chemotherapy with paclitaxel, docetaxel, or vinflunine. The coprimary end points were overall survival and progression-free survival, which were assessed among all patients and among patients who had a tumor PD-1 ligand (PD-L1) combined positive score (the percentage of PD-L1-expressing tumor and infiltrating immune cells relative to the total number of tumor cells) of 10% or more.
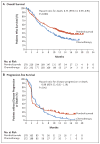
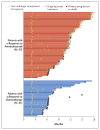
Results: The median overall survival in the total population was 10.3 months (95% confidence interval [CI], 8.0 to 11.8) in the pembrolizumab group, as compared with 7.4 months (95% CI, 6.1 to 8.3) in the chemotherapy group (hazard ratio for death, 0.73; 95% CI, 0.59 to 0.91; P=0.002). The median overall survival among patients who had a tumor PD-L1 combined positive score of 10% or more was 8.0 months (95% CI, 5.0 to 12.3) in the pembrolizumab group, as compared with 5.2 months (95% CI, 4.0 to 7.4) in the chemotherapy group (hazard ratio, 0.57; 95% CI, 0.37 to 0.88; P=0.005). There was no significant between-group difference in the duration of progression-free survival in the total population (hazard ratio for death or disease progression, 0.98; 95% CI, 0.81 to 1.19; P=0.42) or among patients who had a tumor PD-L1 combined positive score of 10% or more (hazard ratio, 0.89; 95% CI, 0.61 to 1.28; P=0.24). Fewer treatment-related adverse events of any grade were reported in the pembrolizumab group than in the chemotherapy group (60.9% vs. 90.2%); there were also fewer events of grade 3, 4, or 5 severity reported in the pembrolizumab group than in the chemotherapy group (15.0% vs. 49.4%).
Conclusions: Pembrolizumab was associated with significantly longer overall survival (by approximately 3 months) and with a lower rate of treatment-related adverse events than chemotherapy as second-line therapy for platinum-refractory advanced urothelial carcinoma. (Funded by Merck; KEYNOTE-045 ClinicalTrials.gov number, NCT02256436 .).
Figures

Comment in
-
Bladder cancer: Pembrolizumab is superior to chemotherapy.Nat Rev Urol. 2017 May;14(5):261. doi: 10.1038/nrurol.2017.38. Epub 2017 Mar 7. Nat Rev Urol. 2017. PMID: 28266514 No abstract available.
-
Urological cancer: Unravelling intertwined second-line options.Nat Rev Clin Oncol. 2017 Mar 20;14(4):197. doi: 10.1038/nrclinonc.2017.38. Nat Rev Clin Oncol. 2017. PMID: 28316332 No abstract available.
-
Expanding Immunotherapy Options for Bladder Cancer: Commentary on: Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma.Urology. 2017 Aug;106:1-2. doi: 10.1016/j.urology.2017.04.001. Epub 2017 Apr 25. Urology. 2017. PMID: 28454987 No abstract available.
-
Pembrolizumab for Advanced Urothelial Carcinoma.N Engl J Med. 2017 Jun 8;376(23):2302. doi: 10.1056/NEJMc1704612. N Engl J Med. 2017. PMID: 28591524 No abstract available.
-
Pembrolizumab for Advanced Urothelial Carcinoma.N Engl J Med. 2017 Jun 8;376(23):2303-2304. doi: 10.1056/NEJMc1704612. N Engl J Med. 2017. PMID: 28591525 No abstract available.
-
Pembrolizumab for Advanced Urothelial Carcinoma.N Engl J Med. 2017 Jun 8;376(23):2302-3. doi: 10.1056/NEJMc1704612. N Engl J Med. 2017. PMID: 28594151 No abstract available.
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Seront E, Machiels JP. Molecular biology and targeted therapies for urothelial carcinoma. Cancer Treat Rev. 2015;41:341–53. - PubMed
-
- Oing C, Rink M, Oechsle K, Seidel C, von Amsberg G, Bokemeyer C. Second line chemotherapy for advanced and metastatic urothelial carcinoma: vinflunine and beyond — a comprehensive review of the current literature. J Urol. 2016;195:254–63. - PubMed
-
- Raggi D, Miceli R, Sonpavde G, et al. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27:49–61. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
