Improving Registration Robustness for Image-Guided Liver Surgery in a Novel Human-to-Phantom Data Framework
- PMID: 28212080
- PMCID: PMC5757161
- DOI: 10.1109/TMI.2017.2668842
Improving Registration Robustness for Image-Guided Liver Surgery in a Novel Human-to-Phantom Data Framework
Abstract
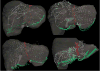
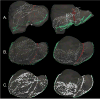
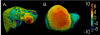
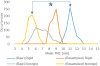
In open image-guided liver surgery (IGLS), a sparse representation of the intraoperative organ surface can be acquired to drive image-to-physical registration. We hypothesize that uncharacterized error induced by variation in the collection patterns of organ surface data limits the accuracy and robustness of an IGLS registration. Clinical validation of such registration methods is challenged due to the difficulty in obtaining data representative of the true state of organ deformation. We propose a novel human-to-phantom validation framework that transforms surface collection patterns from in vivo IGLS procedures (n = 13) onto a well-characterized hepatic deformation phantom for the purpose of validating surface-driven, volumetric nonrigid registration methods. An important feature of the approach is that it centers on combining workflow-realistic data acquisition and surgical deformations that are appropriate in behavior and magnitude. Using the approach, we investigate volumetric target registration error (TRE) with both current rigid IGLS and our improved nonrigid registration methods. Additionally, we introduce a spatial data resampling approach to mitigate the workflow-sensitive sampling problem. Using our human-to-phantom approach, TRE after routine rigid registration was 10.9 ± 0.6 mm with a signed closest point distance associated with residual surface fit in the range of ±10 mm, highly representative of open liver resections. After applying our novel resampling strategy and improved deformation correction method, TRE was reduced by 51%, i.e., a TRE of 5.3 ± 0.5 mm. This paper reported herein realizes a novel tractable approach for the validation of image-to-physical registration methods and demonstrates promising results for our correction method.
Figures



References
-
- Maier-Hein L, et al. Optical techniques for 3D surface reconstruction in computer-assisted laparoscopic surgery. Med Image Anal. 2013;17(8):974–996. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

