Defining Clinical Response Criteria and Early Response Criteria for Precision Oncology: Current State-of-the-Art and Future Perspectives
- PMID: 28212290
- PMCID: PMC5373019
- DOI: 10.3390/diagnostics7010010
Defining Clinical Response Criteria and Early Response Criteria for Precision Oncology: Current State-of-the-Art and Future Perspectives
Abstract
In this era of precision oncology, there has been an exponential growth in the armamentarium of genomically targeted therapies and immunotherapies. Evaluating early responses to precision therapy is essential for "go" versus "no go" decisions for these molecularly targeted drugs and agents that arm the immune system. Many different response assessment criteria exist for use in solid tumors and lymphomas. We reviewed the literature using the Medline/PubMed database for keywords "response assessment" and various known response assessment criteria published up to 2016. In this article we review the commonly used response assessment criteria. We present a decision tree to facilitate selection of appropriate criteria. We also suggest methods for standardization of various response assessment criteria. The relevant response assessment criteria were further studied for rational of development, key features, proposed use and acceptance by various entities. We also discuss early response evaluation and provide specific case studies of early response to targeted therapy. With high-throughput, advanced computing programs and digital data-mining it is now possible to acquire vast amount of high quality imaging data opening up a new field of "omics in radiology"-radiomics that complements genomics for personalized medicine. Radiomics is rapidly evolving and is still in the research arena. This cutting-edge technology is poised to move soon to the mainstream clinical arena. Novel agents with new mechanisms of action require advanced molecular imaging as imaging biomarkers. There is an urgent need for development of standardized early response assessment criteria for evaluation of response to precision therapy.
Keywords: EASL; MDA criteria; PERCIST; RANO criteri; RECICL; RECIST; WHO criteria; irRC; mRECIST.
Conflict of interest statement
Vivek Subbiah receives research funding for clinical trials from Novartis, Bayer, GSK, Nanocarrier, Vegenics, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, Pharmamar, D3, Pfizer, Multivir, Amgen, Abbvie, Bluprint medicines and Roche/ Genentech. All the other authors declare no relevant conflicts of interest.
Figures



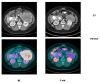
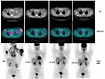
References
-
- World Health Organization . WHO Handbook for Reporting Results of Cancer Treatment. World Health Organization; Geneva, Switzerland: 1979.
-
- Forner A., Ayuso C., Varela M., Rimola J., Hessheimer A.J., de Lope C.R., Reig M., Bianchi L., Llovet J.M., Bruix J. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: Are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616–623. doi: 10.1002/cncr.24050. - DOI - PubMed
-
- Therasse P., Arbuck S.G., Eisenhauer E.A., Wanders J., Kaplan R.S., Rubinstein L., Verweij J., Van Glabbeke M., van Oosterom A.T., Christian M.C., et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

