Methylglyoxal-Derived Advanced Glycation Endproducts in Multiple Sclerosis
- PMID: 28212304
- PMCID: PMC5343955
- DOI: 10.3390/ijms18020421
Methylglyoxal-Derived Advanced Glycation Endproducts in Multiple Sclerosis
Abstract
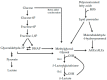
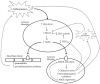
Multiple sclerosis (MS) is a demyelinating disease of the central nervous system (CNS). The activation of inflammatory cells is crucial for the development of MS and is shown to induce intracellular glycolytic metabolism in pro-inflammatory microglia and macrophages, as well as CNS-resident astrocytes. Advanced glycation endproducts (AGEs) are stable endproducts formed by a reaction of the dicarbonyl compounds methylglyoxal (MGO) and glyoxal (GO) with amino acids in proteins, during glycolysis. This suggests that, in MS, MGO-derived AGEs are formed in glycolysis-driven cells. MGO and MGO-derived AGEs can further activate inflammatory cells by binding to the receptor for advanced glycation endproducts (RAGE). Recent studies have revealed that AGEs are increased in the plasma and brain of MS patients. Therefore, AGEs might contribute to the inflammatory status in MS. Moreover, the main detoxification system of dicarbonyl compounds, the glyoxalase system, seems to be affected in MS patients, which may contribute to high MGO-derived AGE levels. Altogether, evidence is emerging for a contributing role of AGEs in the pathology of MS. In this review, we provide an overview of the current knowledge on the involvement of AGEs in MS.
Keywords: advanced glycation endproducts; glyoxalase system; methylglyoxal; multiple sclerosis; receptor for advanced glycation endproduct.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

