The p53 isoform delta133p53ß regulates cancer cell apoptosis in a RhoB-dependent manner
- PMID: 28212429
- PMCID: PMC5315499
- DOI: 10.1371/journal.pone.0172125
The p53 isoform delta133p53ß regulates cancer cell apoptosis in a RhoB-dependent manner
Erratum in
-
Correction: The p53 isoform delta133p53ß regulates cancer cell apoptosis in a RhoB-dependent manner.PLoS One. 2017 Apr 6;12(4):e0175607. doi: 10.1371/journal.pone.0175607. eCollection 2017. PLoS One. 2017. PMID: 28384271 Free PMC article.
Abstract
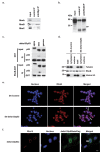
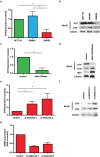
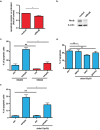
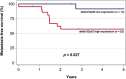
The TP53 gene plays essential roles in cancer. Conventionally, wild type (WT) p53 is thought to prevent cancer development and metastasis formation, while mutant p53 has transforming abilities. However, clinical studies failed to establish p53 mutation status as an unequivocal predictive or prognostic factor of cancer progression. The recent discovery of p53 isoforms that can differentially regulate cell cycle arrest and apoptosis suggests that their expression, rather than p53 mutations, could be a more clinically relevant biomarker in patients with cancer. In this study, we show that the p53 isoform delta133p53ß is involved in regulating the apoptotic response in colorectal cancer cell lines. We first demonstrate delta133p53ß association with the small GTPase RhoB, a well-described anti-apoptotic protein. We then show that, by inhibiting RhoB activity, delta133p53ß protects cells from camptothecin-induced apoptosis. Moreover, we found that high delta133p53 mRNA expression levels are correlated with higher risk of recurrence in a series of patients with locally advanced rectal cancer (n = 36). Our findings describe how a WT TP53 isoform can act as an oncogene and add a new layer to the already complex p53 signaling network.
Conflict of interest statement
Figures
References
-
- Gottlieb TM, Oren M (1998) p53 and apoptosis. Seminars in cancer biology 8: 359–368. - PubMed
-
- Symonds H, Krall L, Remington L, Saenz-Robles M, Lowe S, Jacks T, et al. (1994) p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell 78: 703–711. - PubMed
-
- Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, et al. (1999) Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284: 156–159. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

