Genetically encoded ratiometric fluorescent thermometer with wide range and rapid response
- PMID: 28212432
- PMCID: PMC5315395
- DOI: 10.1371/journal.pone.0172344
Genetically encoded ratiometric fluorescent thermometer with wide range and rapid response
Abstract
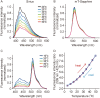
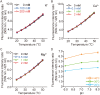
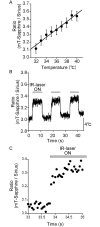
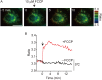
Temperature is a fundamental physical parameter that plays an important role in biological reactions and events. Although thermometers developed previously have been used to investigate several important phenomena, such as heterogeneous temperature distribution in a single living cell and heat generation in mitochondria, the development of a thermometer with a sensitivity over a wide temperature range and rapid response is still desired to quantify temperature change in not only homeotherms but also poikilotherms from the cellular level to in vivo. To overcome the weaknesses of the conventional thermometers, such as a limitation of applicable species and a low temporal resolution, owing to the narrow temperature range of sensitivity and the thermometry method, respectively, we developed a genetically encoded ratiometric fluorescent temperature indicator, gTEMP, by using two fluorescent proteins with different temperature sensitivities. Our thermometric method enabled a fast tracking of the temperature change with a time resolution of 50 ms. We used this method to observe the spatiotemporal temperature change between the cytoplasm and nucleus in cells, and quantified thermogenesis from the mitochondria matrix in a single living cell after stimulation with carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone, which was an uncoupler of oxidative phosphorylation. Moreover, exploiting the wide temperature range of sensitivity from 5°C to 50°C of gTEMP, we monitored the temperature in a living medaka embryo for 15 hours and showed the feasibility of in vivo thermometry in various living species.
Conflict of interest statement
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

