Upregulation of mucin glycoprotein MUC1 in the progression to esophageal adenocarcinoma and therapeutic potential with a targeted photoactive antibody-drug conjugate
- PMID: 28212575
- PMCID: PMC5421911
- DOI: 10.18632/oncotarget.15340
Upregulation of mucin glycoprotein MUC1 in the progression to esophageal adenocarcinoma and therapeutic potential with a targeted photoactive antibody-drug conjugate
Abstract
Background: Mucin glycoprotein 1 (MUC1) is a glycosylated transmembrane protein on epithelial cells. We investigate MUC1 as a therapeutic target in Barrett's epithelium (BE) and esophageal adenocarcinoma (EA) and provide proof of concept for a light based therapy targeting MUC1.
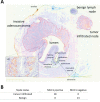
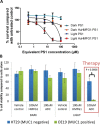
Results: MUC1 was present in 21% and 30% of significantly enriched pathways comparing BE and EA to squamous epithelium respectively. MUC1 gene expression was x2.3 and x2.2 higher in BE (p=<0.001) and EA (p=0.03). MUC1 immunohistochemical expression increased during progression to EA and followed tumor invasion. HuHMFG1 based photosensitive antibody drug conjugates (ADC) showed cell internalization, MUC1 selective and light-dependent cytotoxicity (p=0.0006) and superior toxicity over photosensitizer alone (p=0.0022).
Methods: Gene set enrichment analysis (GSEA) evaluated pathways during BE and EA development and quantified MUC1 gene expression. Immunohistochemistry and flow cytometry evaluated the anti-MUC1 antibody HuHMFG1 in esophageal cells of varying pathological grade. Confocal microscopy examined HuHMFG1 internalization and HuHMFG1 ADCs were created to deliver a MUC1 targeted phototoxic payload.
Conclusions: MUC1 is a promising target in EA. Molecular and light based targeting of MUC1 with a photosensitive ADC is effective in vitro and after development may enable treatment of locoregional tumors endoscopically.
Keywords: Barrett’s esophagus; antibody-drug conjugate; esophageal adenocarcinoma; mucins; photodynamic therapy.
Conflict of interest statement
GY & MD co-founded Antikor BioPharma who provided PS1 used in this study; patent name “Compounds and biological materials and used thereof” and number WO 2010106341 [42]. There are no further patents, products in development or marketed products to declare.
Figures






References
-
- Cancer Research UK Cancer Survival Group. Age-Standardised One-, Five- and Ten-Year Net Survival, Adults (Aged 15-99), England and Wales. 2014. pp. 2010–2011.http://www.cancerresearchuk.org/health-professional/cancer-statistics/st... Available from.
-
- Haidry RJ, Dunn JM, Butt MA, Burnell MG, Gupta A, Green S, Miah H, Smart HL, Bhandari P, Smith LA, Willert R, Fullarton G, Morris J, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic Barrett’s esophagus and early esophageal adenocarcinoma: Outcomes of the UK national halo RFA registry. Gastroenterology. 2013;145:87–95. doi: 10.1053/j.gastro.2013.03.045. - DOI - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

