A mixed methods feasibility study of nicotine-assisted smoking reduction programmes delivered by community pharmacists - The RedPharm study
- PMID: 28212652
- PMCID: PMC5316161
- DOI: 10.1186/s12889-017-4116-z
A mixed methods feasibility study of nicotine-assisted smoking reduction programmes delivered by community pharmacists - The RedPharm study
Erratum in
-
Erratum to: A mixed methods feasibility study of nicotine-assisted smoking reduction programmes delivered by community pharmacists - The RedPharm study.BMC Public Health. 2017 Mar 31;17(1):288. doi: 10.1186/s12889-017-4204-0. BMC Public Health. 2017. PMID: 28363278 Free PMC article. No abstract available.
Abstract
Background: Pivotal trials have established that, among people who have no immediate intention to quit smoking, nicotine replacement therapy (NRT) helps people reduce and eventually stop smoking. The prime aim of this trial was to investigate the feasibility of implementing such a programme in community pharmacies. In addition, we investigated the effectiveness of providing behavioural support compared with self-help methods and of shorter compared with standard length reduction programmes.
Methods: Pharmacists were trained to deliver a smoking reduction programme and opportunistically invite people to participate in the programme. In a 2 × 2 factorial design, eligible volunteers were randomised to either receive in-person behavioural support or a self-help booklet. In both cases, participants were supported to set targets to reduce their smoking and use behavioural techniques to assist reduction. In addition, participants were randomised to cut down and stop over 4 weeks or over 16 weeks, but in either case continue NRT for up to nine months. We assessed uptake and adherence to the programme and smoking cessation four weeks and six months after a quit day and reduction in the three months following programme end and incorporated a qualitative processes assessment.
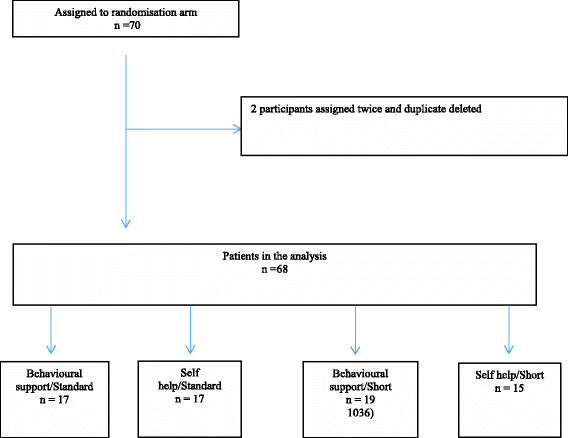
Results: Only 68 of the planned 160 smokers could be recruited. Pharmacists were deterred by the bureaucracy of trial enrolment and that many smokers did not return for further support. Pharmacists sometimes subverted the randomisation or provided support to participants in the self-help arm. Smokers stayed in the programme for an average of 6 weeks rather than the 9 months envisaged. Rates of follow-up declined to around 20% of participants by 12 months. There was insufficient evidence to assess whether support or speed of reduction enhanced cessation or reduction but cessation and reduction were less common overall than in the pivotal trials for licensing NRT for this indication.
Conclusions: This programme of smoking reduction and the trial design to assess its effectiveness proved unpopular to potential participants and pharmacists. As a result, the trial produced no evidence on the effectiveness of behavioural support or speed or smoking reduction. A trial of this programme in this context is unfeasible.
Trial registration: ISRCTN 54805841 . Registered 18 March 2010.
Figures
References
-
- West R. Smoking and smoking cessation in England. In: 2012.http://www.smokinginengland.info/latest-statistics/. Accessed 13 Apr 2012.
-
- NICE. NICE public health guidance 10: Smoking cessation service. In.; 2013.
-
- Bauld L, Bell K, McCullough L, Richardson L, Greaves L. The effectiveness of NHS smoking cessation services: a systematic review. J Public Health. 2010;32(1):71-82. doi: 10.1093/pubmed/fdp074. Epub 2009 Jul 28. - PubMed
-
- Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D. Cut down to quit with nicotine replacement therapies (NRT) in smoking cessation: Systematic review of effectiveness and economic analysis. Health Technol Assess 2008;12(2):iii-iv, ix-xi,1-135. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials