Assessing the interruption of the transmission of human helminths with mass drug administration alone: optimizing the design of cluster randomized trials
- PMID: 28212667
- PMCID: PMC5316156
- DOI: 10.1186/s13071-017-1979-x
Assessing the interruption of the transmission of human helminths with mass drug administration alone: optimizing the design of cluster randomized trials
Abstract
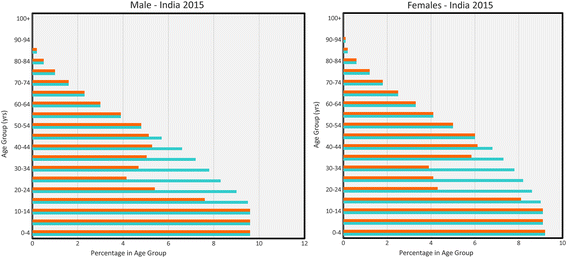
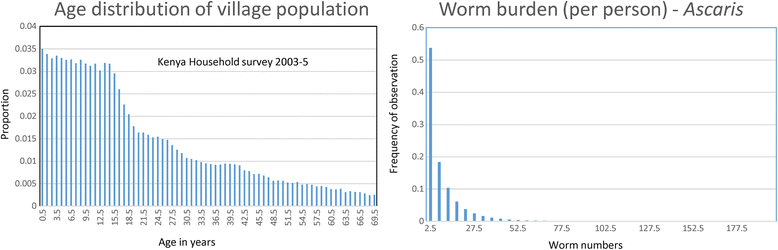
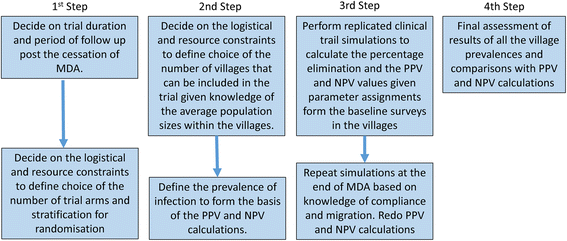
Background: A method is outlined for the use of an individual-based stochastic model of parasite transmission dynamics to assess different designs for a cluster randomized trial in which mass drug administration (MDA) is employed in attempts to eliminate the transmission of soil-transmitted helminths (STH) in defined geographic locations. The hypothesis to be tested is: Can MDA alone interrupt the transmission of STH species in defined settings? Clustering is at a village level and the choice of clusters of villages is stratified by transmission intensity (low, medium and high) and parasite species mix (either Ascaris, Trichuris or hookworm dominant).
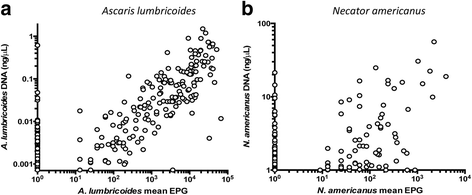
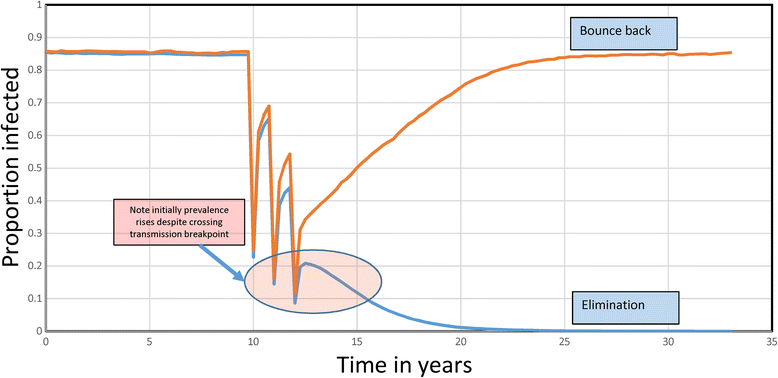
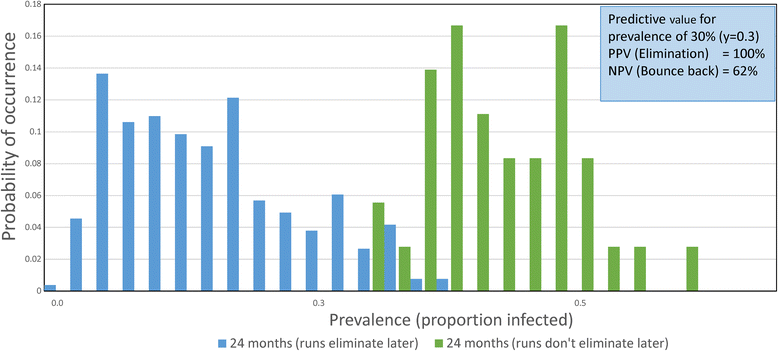
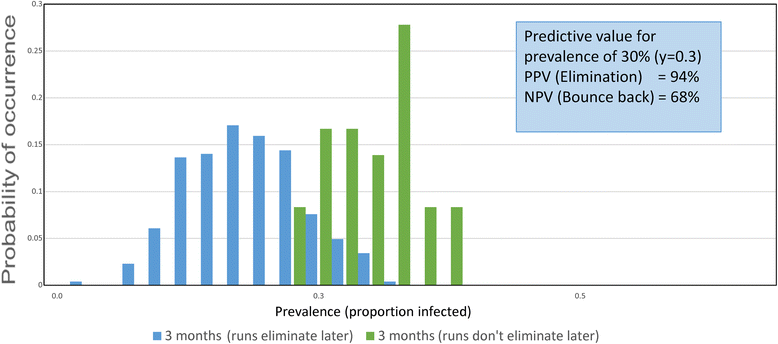
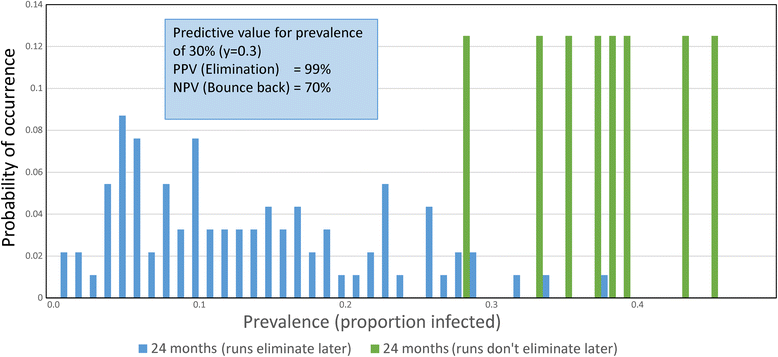
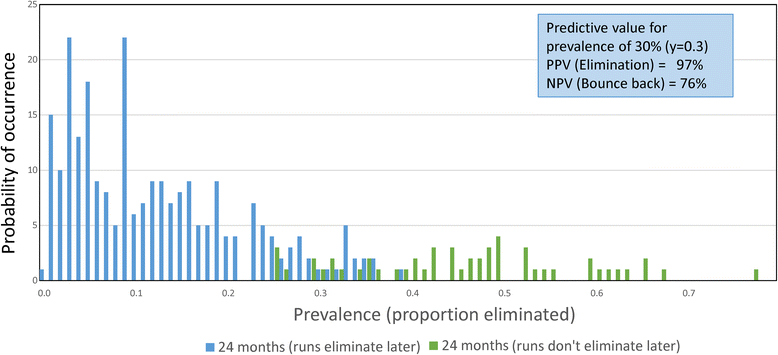
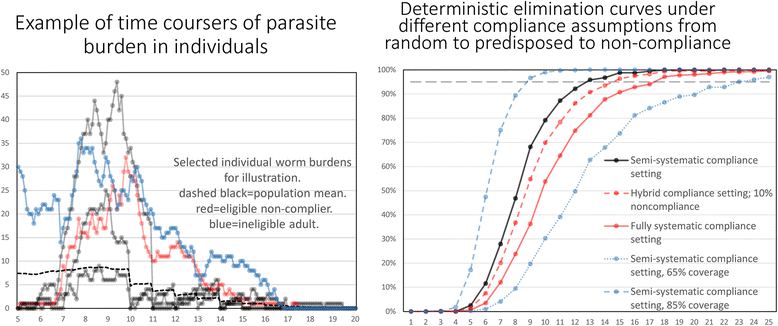
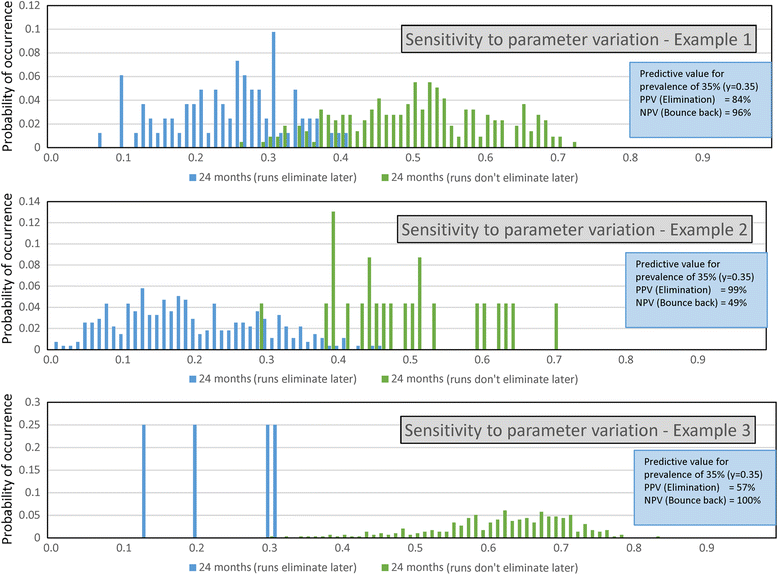
Results: The methodological approach first uses an age-structured deterministic model to predict the MDA coverage required for treating pre-school aged children (Pre-SAC), school aged children (SAC) and adults (Adults) to eliminate transmission (crossing the breakpoint in transmission created by sexual mating in dioecious helminths) with 3 rounds of annual MDA. Stochastic individual-based models are then used to calculate the positive and negative predictive values (PPV and NPV, respectively, for observing elimination or the bounce back of infection) for a defined prevalence of infection 2 years post the cessation of MDA. For the arm only involving the treatment of Pre-SAC and SAC, the failure rate is predicted to be very high (particularly for hookworm-infected villages) unless transmission intensity is very low (R0, or the effective reproductive number R, just above unity in value).
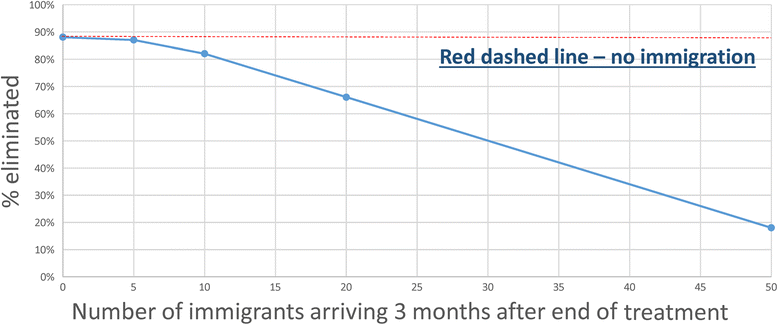
Conclusions: The calculations are designed to consider various trial arms and stratifications; namely, community-based treatment and Pre-SAC and SAC only treatment (the two arms of the trial), different STH transmission settings of low, medium and high, and different STH species mixes. Results are considered in the light of the complications introduced by the choice of statistic to define success or failure, varying adherence to treatment, migration and parameter uncertainty.
Keywords: Cluster randomized trial design; Interrupting transmission; Mass drug administration impact; Soil-transmitted helminths; Stochastic models of transmission.
Figures


References
-
- WHO Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2015. Week Epidem Rec. 2016;91(49–50):585–600. - PubMed
-
- Anderson RM, Turner HC, Truscott JE, Hollingsworth TD, Brooker S. Should the goal for the treatment of Soil Transmitted Helminth (STH) infections be changed from morbidity control in children to community wide transmission elimination? PLoS Negl Trop Dis. 2015;9(8):e0003897. doi: 10.1371/journal.pntd.0003897. - DOI - PMC - PubMed
-
- Brooker SJ, Mwandawiro CS, Haliday KE, Min S, Mcharao C, Gichuki PM, et al. Interrupting transmission of soil-transmitted helminths: a study protocol for cluster randomized trials evaluating alternative treatment strategies and delivery systems in Kenya. BMJ Open. 2015;5:e008950. doi: 10.1136/bmjopen-2015-008950. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

