Desipramine decreases expression of human and murine indoleamine-2,3-dioxygenases
- PMID: 28212884
- PMCID: PMC5382643
- DOI: 10.1016/j.bbi.2017.02.010
Desipramine decreases expression of human and murine indoleamine-2,3-dioxygenases
Abstract
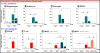
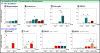
Abundant evidence connects depression symptomology with immune system activation, stress and subsequently elevated levels of kynurenine. Anti-depressants, such as the tricyclic norepinephrine/serotonin reuptake inhibitor desipramine (Desip), were developed under the premise that increasing extracellular neurotransmitter level was the sole mechanism by which they alleviate depressive symptomologies. However, evidence suggests that anti-depressants have additional actions that contribute to their therapeutic potential. The Kynurenine Pathway produces tryptophan metabolites that modulate neurotransmitter activity. This recognition identified another putative pathway for anti-depressant targeting. Considering a recognized role of the Kynurenine Pathway in depression, we investigated the potential for Desip to alter expression of rate-limiting enzymes of this pathway: indoleamine-2,3-dioxygenases (Ido1 and Ido2). Mice were administered lipopolysaccharide (LPS) or synthetic glucocorticoid dexamethasone (Dex) with Desip to determine if Desip alters indoleamine-dioxygenase (DO) expression in vivo following a modeled immune and stress response. This work was followed by treating murine and human peripheral blood mononuclear cells (PBMCs) with interferon-gamma (IFNγ) and Desip. In vivo: Desip blocked LPS-induced Ido1 expression in hippocampi, astrocytes, microglia and PBMCs and Ido2 expression by PBMCs. Ex vivo: Desip decreased IFNγ-induced Ido1 and Ido2 expression in murine PBMCs. This effect was directly translatable to the human system as Desip decreased IDO1 and IDO2 expression by human PBMCs. These data demonstrate for the first time that an anti-depressant alters expression of Ido1 and Ido2, identifying a possible new mechanism behind anti-depressant activity. Furthermore, we propose the assessment of PBMCs for anti-depressant responsiveness using IDO expression as a biomarker.
Keywords: Depression; Desipramine; Ido1; Inflammation; Kynurenine pathway; PBMCs.
Published by Elsevier Inc.
Conflict of interest statement
Authors declare that there are no personal or financial conflicts of interest.
Figures
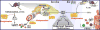




References
-
- Anderson IM. SSRIS versus tricyclic antidepressants in depressed inpatients: a meta-analysis of efficacy and tolerability. Depress Anxiety. 1998;7(Suppl 1):11–17. - PubMed
-
- Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2009;41:467–471. - PubMed
-
- Baranyi A, Meinitzer A, Breitenecker RJ, Amouzadeh-Ghadikolai O, Stauber R, Rothenhausler HB. Quinolinic Acid Responses during Interferon-alpha-Induced Depressive Symptomatology in Patients with Chronic Hepatitis C Infection - A Novel Aspect for Depression and Inflammatory Hypothesis. PLoS One. 2015;10:e0137022. - PMC - PubMed
-
- Basu Mallik S, Mudgal J, Nampoothiri M, Hall S, Dukie SA, Grant G, Rao CM, Arora D. Caffeic acid attenuates lipopolysaccharide-induced sickness behaviour and neuroinflammation in mice. Neurosci Lett. 2016;632:218–223. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

